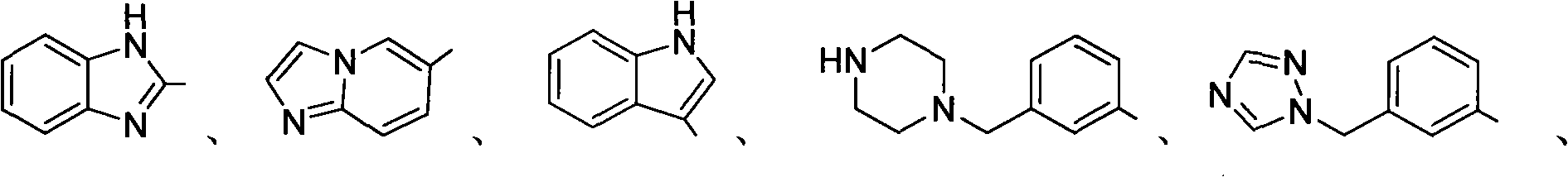
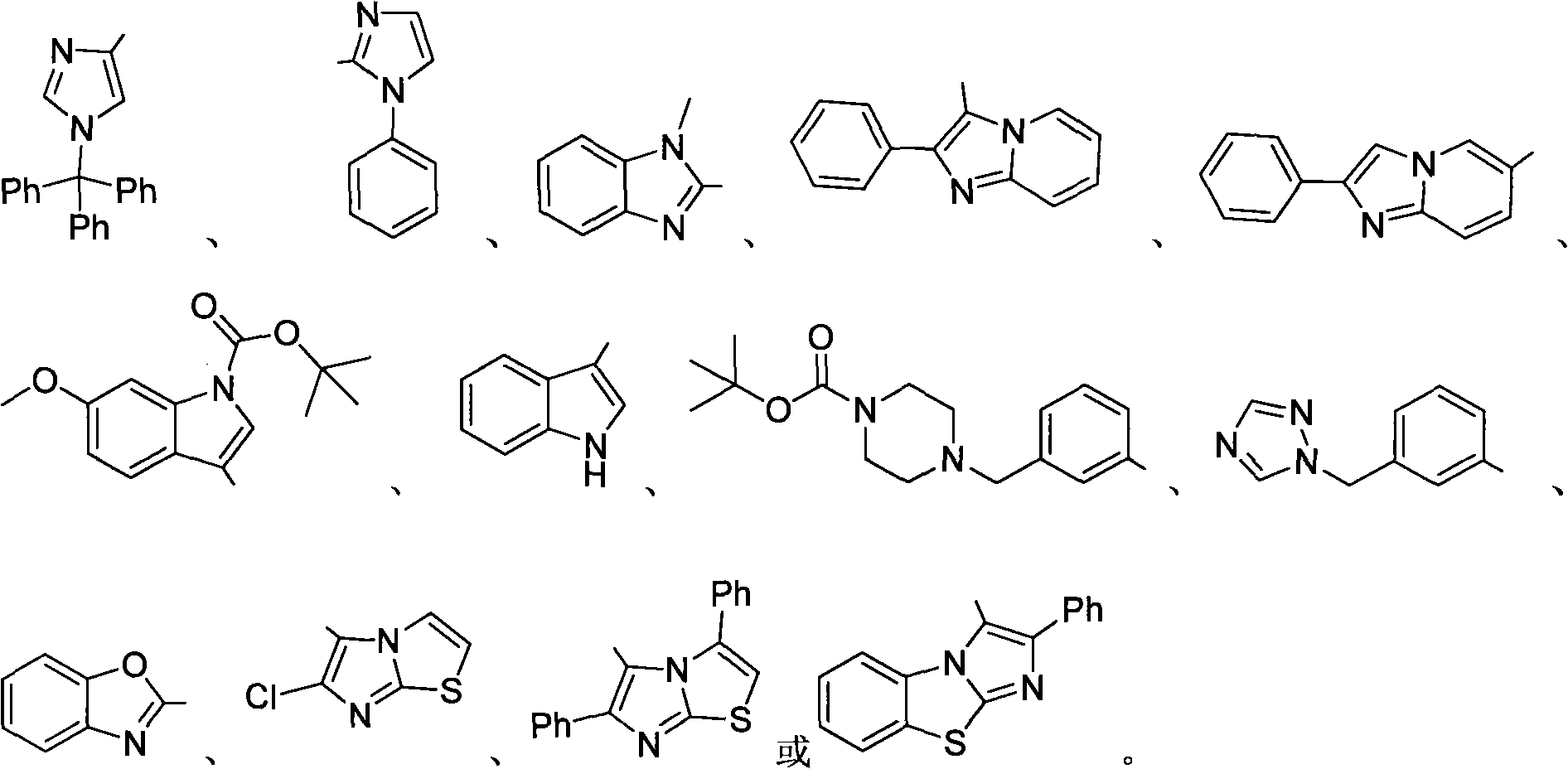
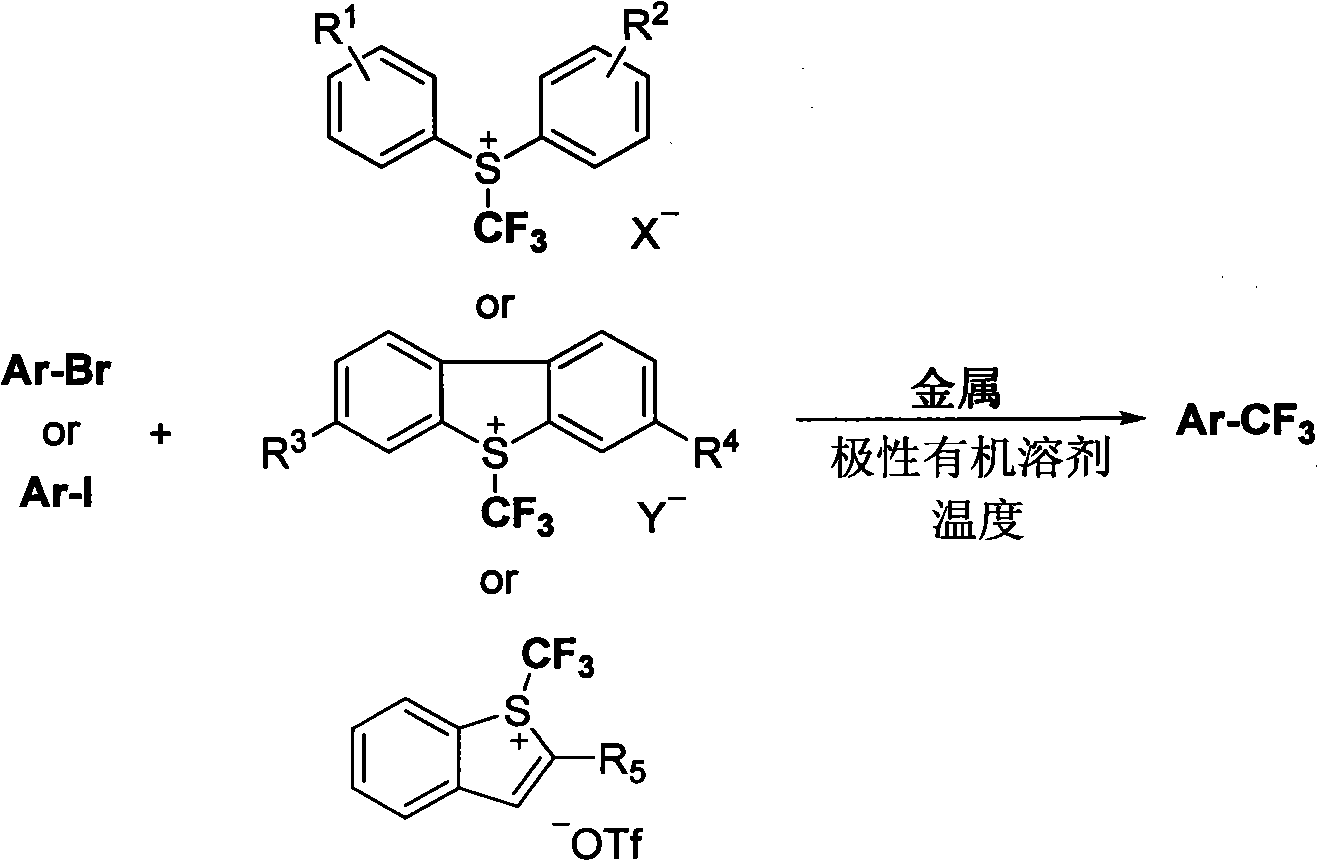
Trifluoromethylation of trifluoromethyl aryl sulfonium salt to heterocyclic compound under metal trigger
A trifluoromethyl aryl, trifluoromethyl metal technology, applied in the field of trifluoromethylation, can solve the problems of high reaction temperature, strong UV initiation, poor reaction selectivity of trifluoromethylation, etc. Mild conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] Example 1: preparation of
[0031] In a 2 mL sealed tube, mix 4-iodopyridine (20.5 mg, 0.1 mmol) and [Ph 2 SCF 3 ] + [OTf] -(Trifluoromethyldiphenylsulfonium salt, 81mg, 0.2mmol) was dissolved in DMF (1mL), copper powder (20mg, 0.3mmol) was added, and then sealed and reacted at 60°C for 11h. Yield of fluorine spectrum: 91%.
[0032] 19 F NMR (DMF): δ-65.4 (s, 3F). GC-MS (m / z): 147.0.
example 2
[0033] Example 2: preparation of
[0034] In a 2 mL sealed tube, mix 3-chloro-6-iodopyridazine (24 mg, 0.1 mmol) and [Ph 2 SCF 3 ] + [OTf] - (Trifluoromethyldiphenylsulfonium salt, 81mg, 0.2mmol) was dissolved in DMF (1mL), copper powder (20mg, 0.3mmol) was added, and the tube was locked for 11h at 60°C. Then the heating was stopped, and after the reaction liquid was cooled, it was diluted with diethyl ether (30 mL), and washed three times with water (3×10 mL). The ether layer was separated. Anhydrous Na 2 SO 4 After drying, evaporate to dryness under reduced pressure. The obtained crude product was separated by column chromatography (n-pentane: ether=5:1) to obtain the product (18 mg), yield: 98%.
[0035] 1 H NMR (CDCl 3 ): δ7.81(d, 1H), 7.74(d, 1H). 19 F NMR (CDCl 3 ): δ-66.5(s, 3F).
example 3
[0036] Example 3: preparation of
[0037] In a sealed 2 mL tube, mix 4-iodo-5-methyl-1-phenyl-1H-pyrazole (28.4 mg, 0.1 mmol) and [Ph 2 SCF 3 ] + [OTf] - (Trifluoromethyldiphenylsulfonium salt, 81mg, 0.2mmol) was dissolved in DMF (1mL), copper powder (20mg, 0.3mmol) was added, and the tube was locked for 11h at 60°C. Then the heating was stopped, and after the reaction liquid was cooled, it was diluted with diethyl ether (30 mL), and washed three times with water (3×10 mL). The ether layer was separated. Anhydrous Na 2 SO 4 Dry and evaporate to dryness under reduced pressure. The obtained crude product was separated by column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain the product (21.6 mg), yield: 95%. 1 H NMR (CDCl 3 ): δ7.80(s, 1H), 7.50(m, 3H), 7.43(m, 2H), 2.41(s, 3H). 19 FNMR (CDCl 3 ): δ-56.6(s, 3F). 13 C NMR (CDCl 3 ): δ138.7, 137.7(q, J=2.9Hz), 129.3, 128.8, 125.6, 123.3(q, J=266Hz), 112.3(q, J=37.4Hz), 10.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com