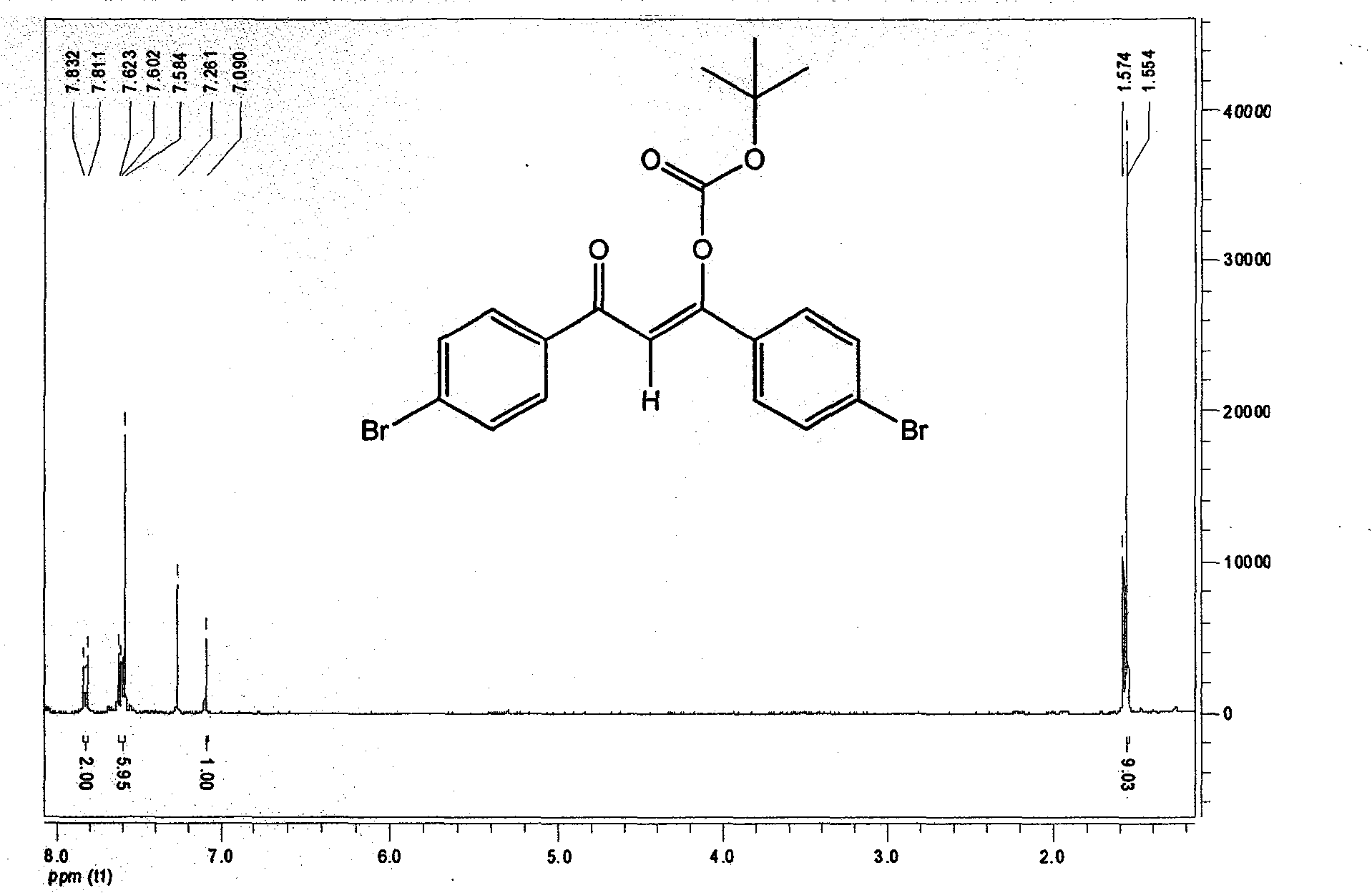
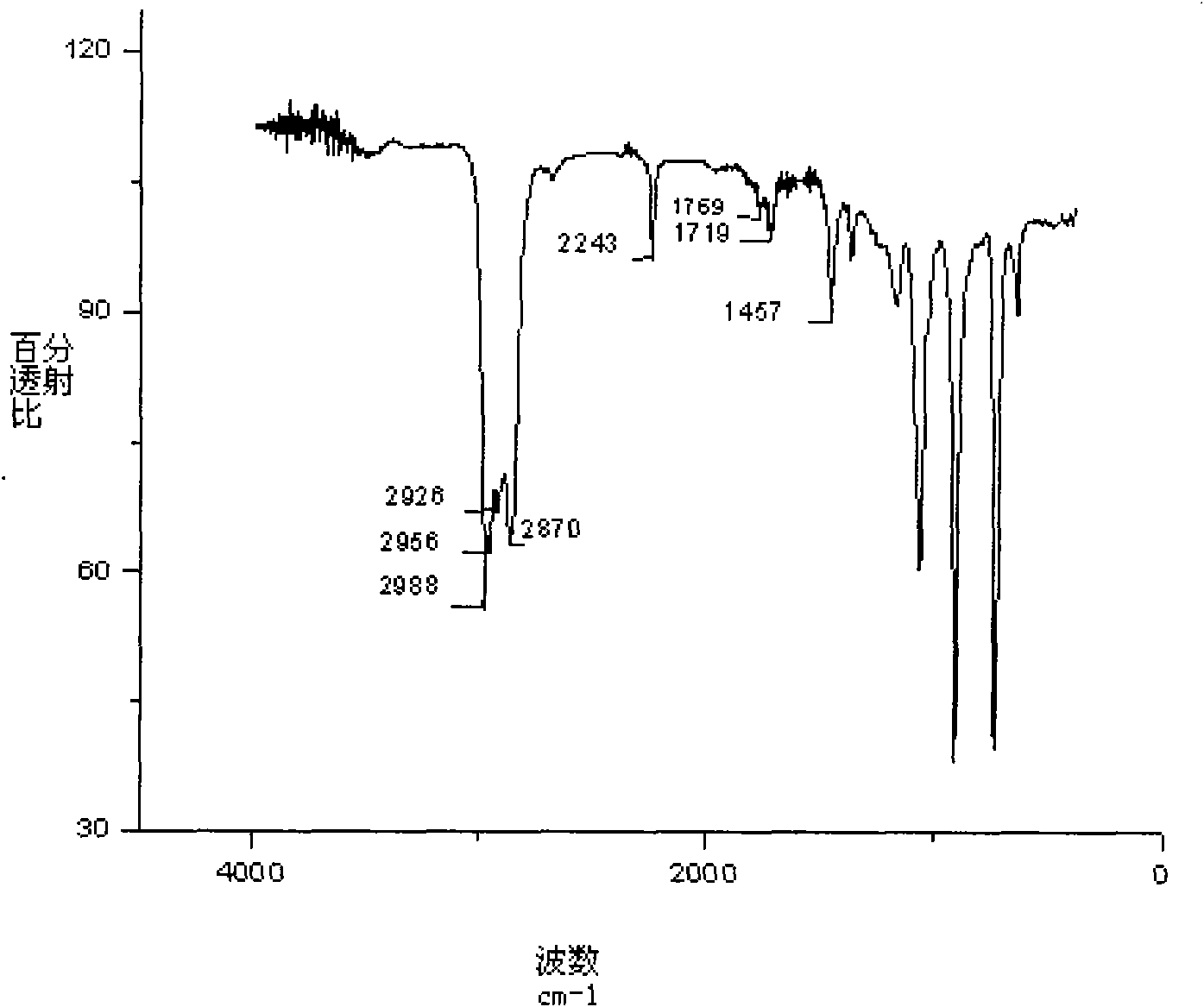
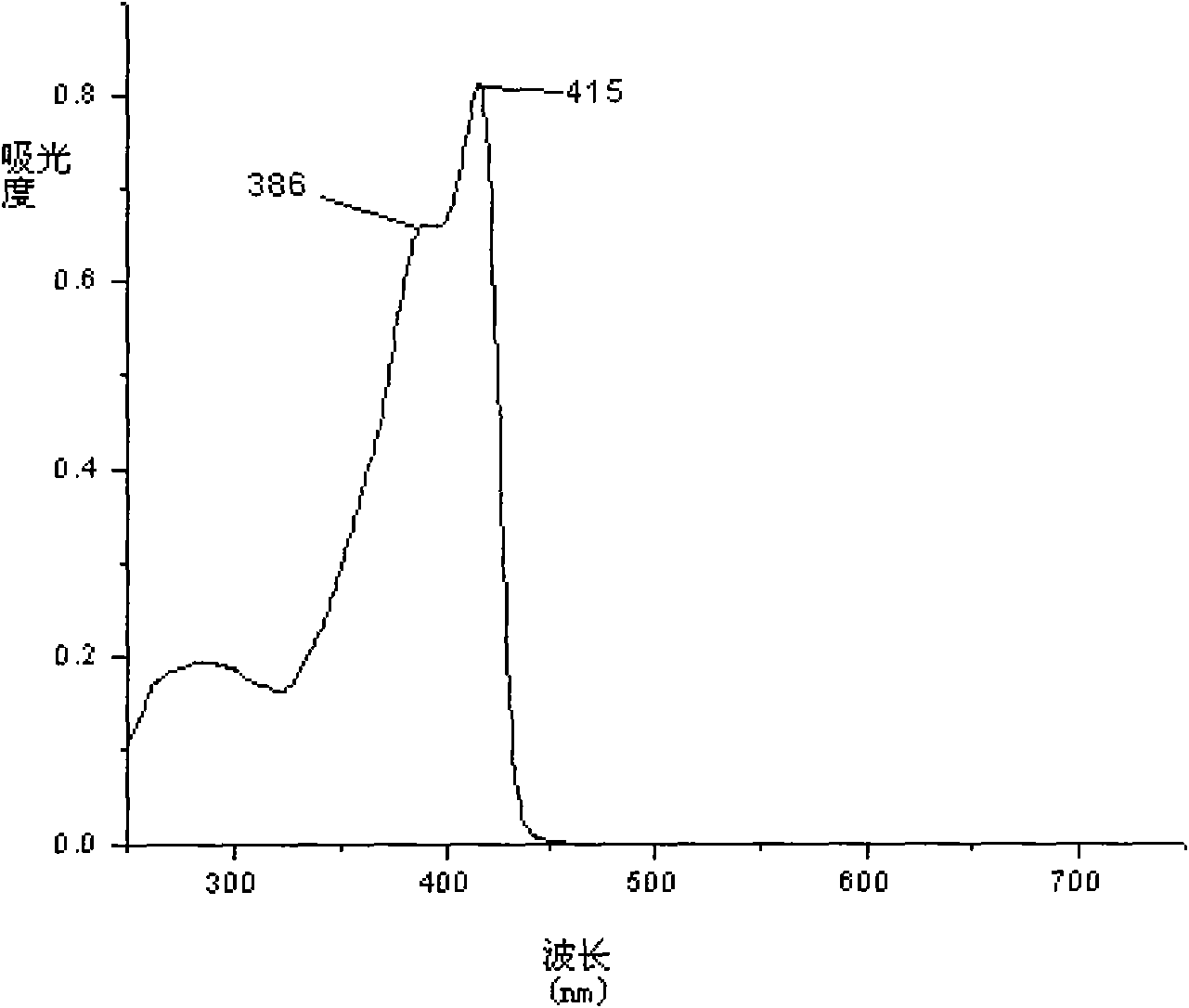
Conjugated micro molecules or conjugated polymer with beta-diketone-containing main chain and preparation method thereof
A technology of conjugated polymers and small molecules, which is applied in the preparation of organic carbonates, organic chemistry, etc., can solve the problems that the coupling reaction cannot be carried out smoothly, and achieve the effects of strong coordination ability, non-toxic effect, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0045] Add 9,9-di-n-octyl-2-boronate fluorene, and Boc-protected bis(4-bromo)dibenzoylmethane into a two-necked flask equipped with a stirring bar. The above-mentioned flask was dried before use, sealed and evacuated for nitrogen. High-purity nitrogen is treated in a strict water-free and oxygen-free device before use. The catalyst (PPh 3 ) 4 Pd(0) (0.5-2.0 mol%), add toluene and triethylamine with a syringe. Toluene and triethylamine are strictly purified before use to remove some impurities and water. Under the condition of vigorous stirring and avoiding light, the reaction was refluxed. The progress of the reaction was monitored by TLC. After the reaction, the solvent was removed by rotary evaporation and purified by silica gel column chromatography.
example 2
[0047]Add 9,9-di-n-octyl-2-ethynylfluorene and Boc-protected bis(4-bromo)dibenzoylmethane into a two-necked flask equipped with a stirring bar. The above-mentioned flask was dried before use, sealed and evacuated for nitrogen. High-purity nitrogen must be treated in a strict anhydrous and oxygen-free device before use. The catalyst (PPh 3 ) 4 Pd(0) (1.0-3.0 mol%), CuI (3.0-5.0 mol%), tetrahydrofuran and triethylamine were added by syringe. Tetrahydrofuran and triethylamine must be strictly purified before use to remove some impurities and water. The reaction was stirred in the dark and at room temperature. The progress of the reaction was monitored by TLC. After the reaction, the solvent was removed by rotary evaporation and purified by silica gel column chromatography.
[0048] Synthesis of Conjugated Polymers Containing β-Diketones
example 3
[0050] 9,9-di-n-octyl-2,7-diborate fluorene, bis(4-bromo)dibenzoylmethane after Boc protection, 9,9-dioctyl-2,7 -Dibromofluorene is fed into a single-necked flask equipped with a stirring bar in a certain molar ratio. The above-mentioned flask was dried before use, sealed and evacuated for nitrogen. High-purity nitrogen must be treated in a strict anhydrous and oxygen-free device before use. The catalyst (PPh 3 ) 4 Pd(0) (0.5-2.0 mol%), add toluene and triethylamine with a syringe. Toluene and triethylamine must be strictly purified before use to remove some impurities and water. Under the condition of vigorous stirring and avoiding light, the reaction was refluxed for 3 days. After the reaction was complete, it was cooled and the reaction mixture was concentrated, precipitated in methanol, and filtered. Stir vigorously in a mixed solution of hydrazine hydrate:toluene=1:1 for 24h to remove the residual catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com