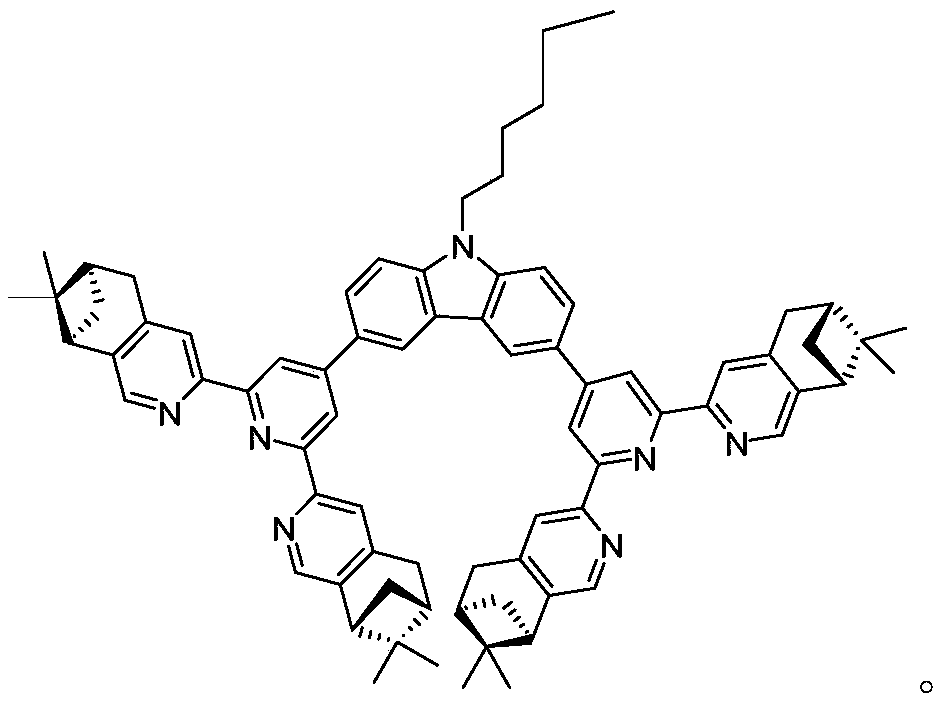
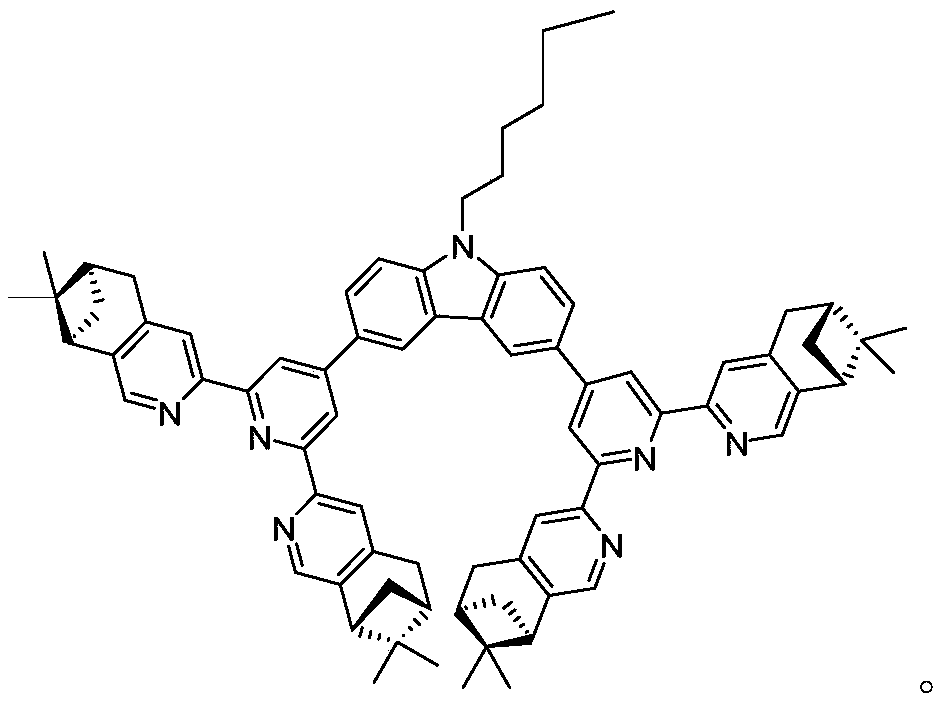
Pinene condensed chiral terpyridyl bidentate compound and preparation method thereof
A technology for terpyridine and compound, which is applied in the field of synthesis and preparation of bidentate compounds, can solve the problems of few reports and researches on tridentate ligands, and achieves the effects of good coordination ability, simple operation, and cheap and easy-to-obtain raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation method of the pinene-fused chiral terpyridine bidentate ligand compound of this embodiment is as follows:
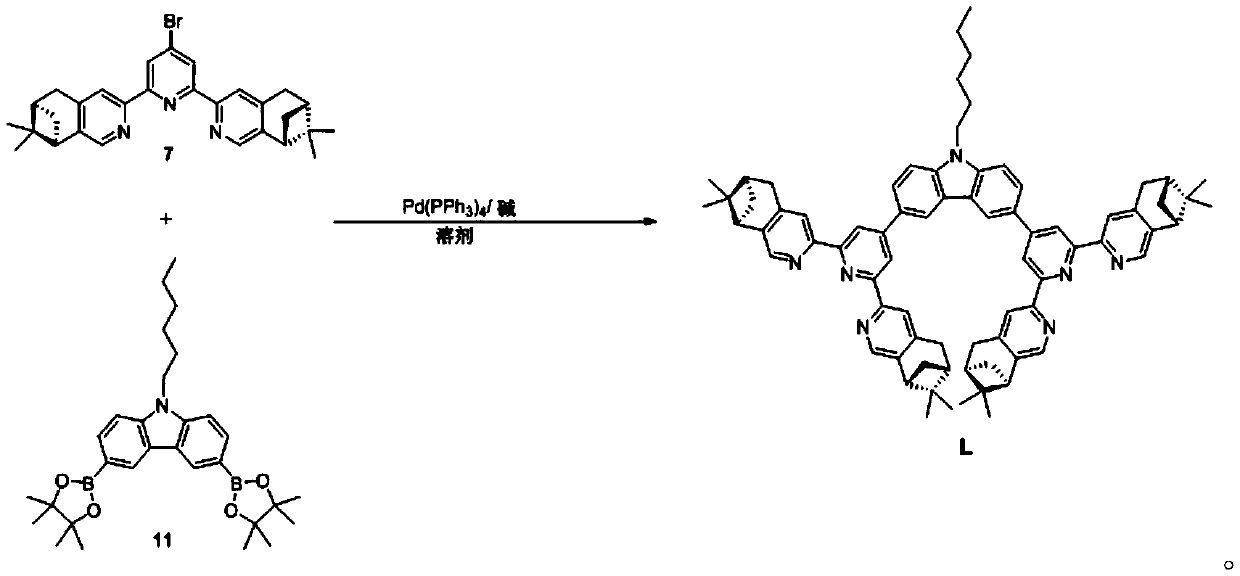
[0021] Under the protection of inert gas, into a 50mL Shrek bottle, add compound 11 (150mg, 0.3mmol), chiral terpyridine 7 (450mg, 0.9mmol), Pd(PPh 3 ) 4 (35mg, 0.03mmol), Na 2 CO 3 (320mg, 3mmol), toluene (10mL), tert-butanol (4mL) and deionized water (4mL), flush three times and seal. The reaction was placed in an oil bath and reacted at 80°C for 48h. After the reaction was completed, cool to room temperature, dissolve the solid with dichloromethane, and wash the organic phase with 50 mL of deionized water. The organic phase was dried over anhydrous sodium sulfate, filtered and the solvent was removed in vacuo. Afterwards, the crude product was purified by column chromatography (eluent: dichloromethane, dichloromethane / methanol=100:0.6) and further purified by column chromatography (eluent: chloroform) to obtain 140 mg of a brown-yellow so...
Embodiment 2
[0024] The synthetic route of chiral terpyridine 7 is as follows:
[0025]
[0026] Synthesis of compound 1
[0027] Install a reflux tube, a drying tube and a hollow plug on a 1000mL three-neck round bottom flask, and install a calcium chloride drying tube on the upper end of the reflux tube. Weigh metallic sodium (13.8g, 0.6mol) and cut it into small pieces, and add it into a three-necked flask containing absolute ethanol (300mL, 5mol) in six to seven times. After the metal sodium and ethanol react completely and cool to room temperature, replace the reflux tube with a constant pressure dropping funnel. At room temperature, a mixed solution of anhydrous acetone (22.8mL, 0.3mol) and diethyl oxalate (86.4mL, 0.6mol) was added dropwise to sodium ethoxide / ethanol, a yellow precipitate formed during the dropwise addition. When remaining 1 / 4, transfer to 60°C oil bath until the dropwise addition is complete. During this process, the aforementioned yellow solid dissolved and ...
Embodiment 3
[0056] Boric acid pinacol ester compound 11 is 9-hexyl-3,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) The synthetic route of -9H-carbazole is as follows:
[0057]
[0058] Synthesis of compound 9
[0059] Compound 8 (3.30 g, 20 mmol) and KOH (2.96 g, 52.8 mmol) were dissolved in DMF (40 mL) and stirred for 60 min at room temperature. Then, add n-C dropwise 6 h 13 Br (3.30 g, 20 mmol), and the reaction mixture was stirred at room temperature for 48 h. Afterwards, the mixture was poured into deionized water and extracted with chloroform, and the organic phase was dried over anhydrous sodium sulfate. The solvent was removed by distillation under reduced pressure, and the crude product was purified by silica gel column chromatography with n-hexane to obtain compound 9 with a yield of 90.9%. The product is a known compound, the 1 H NMR confirmed.
[0060] 9-hexyl-9H-carbazole (9)
[0061]Yellow solid; Mp 61-62℃;
[0062] 1 H NMR (400MHz, CDCl 3 )(δ,ppm)8.10(d,J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com