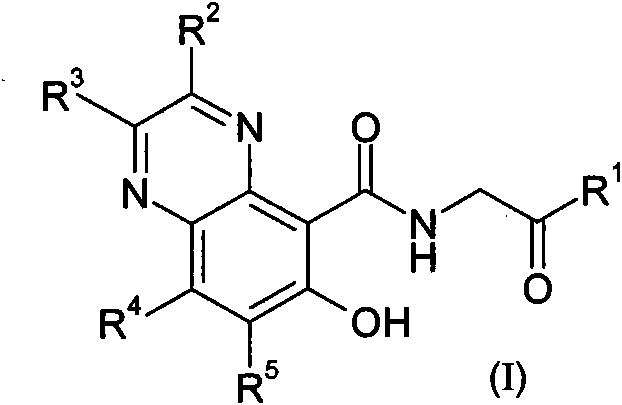
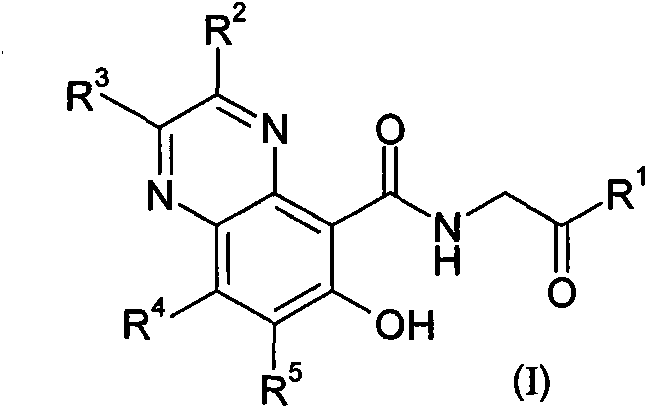
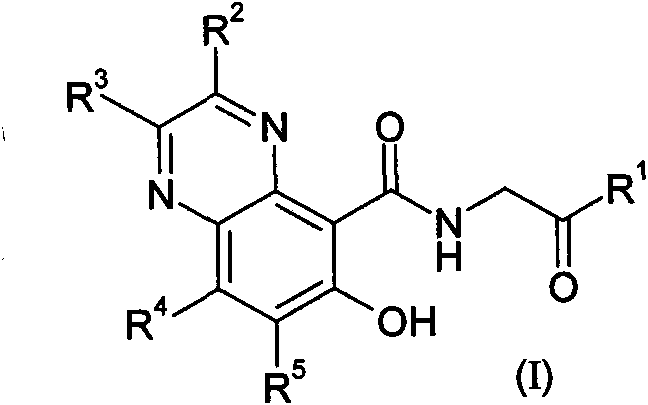
Prolyl hydroxylase inhibitors
A compound and solvate technology, applied in the field of quinoxaline-5-carboxamide derivatives, can solve problems such as increasing Epo production and increasing HIF-α levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0248] Exemplary preparation method
[0249] Program
[0250] Included within the present invention are methods of synthesizing compounds according to Schemes 1-5:
[0251] plan 1
[0252]
[0253] a) fuming nitric acid, concentrated sulfuric acid, heated; b) ammonia (ammonium hydroxide), ethanol; c) sodium hydride or sodium methoxide, methanol; d) H 2 , Pd / C, ethyl acetate, or i. bromine, acetic acid or dichloromethane, ii. tin(II) chloride dihydrate, ethanol, acetonitrile, or H 2 , Pd / C, ethyl acetate, then R 2 C(O)C(O)R3 , acetonitrile / water or methanol, heating or microwave radiation; e) boron tribromide, dichloromethane; f) ethyl glycine hydrochloride, triethylamine or diisopropylethylamine, HATU or PyBOP, N, N-Dimethylformamide or dichloromethane; g) NaOH, ethanol or THF / methanol.
[0254] Scenario 2
[0255]
[0256] a) Phosphorus oxychloride, heating b) Boron tribromide, dichloromethane; c) Glycine ethyl ester hydrochloride, triethylamine or diisopropylethy...
Embodiment 1
[0268]
[0269] N-[(6-Hydroxy-3-phenyl-5-quinoxalinyl)carbonyl]glycine
[0270] 1a) Methyl 2-amino-6-fluoro-3-nitrobenzoate
[0271] To fuming nitric acid (3.87 mL, 86.6 mmol) was slowly added concentrated sulfuric acid (7.27 mL, 136.4 mmol) at 0°C. After stirring for 5 minutes, methyl 2,6-difluorobenzoate (3.90 mL, 29.0 mmol) was added and the reaction mixture was allowed to warm to ambient temperature. After 30 minutes, the reaction mixture was poured into ice-water and extracted three times with dichloromethane. The combined organic fractions were washed with saturated aqueous sodium bicarbonate solution over MgSO 4 Dry, filter, and concentrate in vacuo to give a colorless oil. MS(ES+)m / e 218[M+H] + . On standing, the oil solidified to a white solid, which was dissolved in ethanol (50.0 mL) and treated with ammonia (1.0 mL, 29% in water) at ambient temperature. After 4 hours, additional ammonia (0.8 mL, 29% in water) was added and the reaction mixture was stirr...
Embodiment 2
[0285]
[0286] N-[(6-Hydroxy-3-methyl-5-quinoxalinyl)carbonyl]glycine
[0287] 2a) Methyl 3-methyl-6-(methoxy)-5-quinoxalinecarboxylate
[0288] To a solution of the compound from Example 1 b) (0.307 g, 1.36 mmol) in ethyl acetate (25.0 mL) was added 10% palladium on carbon (0.072 g, 0.068 mmol), and the reactor was then evacuated and purged with nitrogen. The reduction was carried out under 50 psi hydrogen overnight using a Parr shaker. Pass the reaction mixture through Filter, wash with ethyl acetate, and concentrate in vacuo. A suspension of the resulting yellow oil in water (15.0 mL) and acetonitrile (5.0 mL) was treated with methylglyoxal (40 wt % in water) (0.245 g, 1.36 mmol) and heated at 60 °C for 1 h . After cooling, the reaction mixture was diluted with brine and extracted three times with ethyl acetate. The combined organic layers were subjected to MgSO 4 Dry, filter, and concentrate in vacuo to afford the title compound (0.273 g, 87%) as an orange s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com