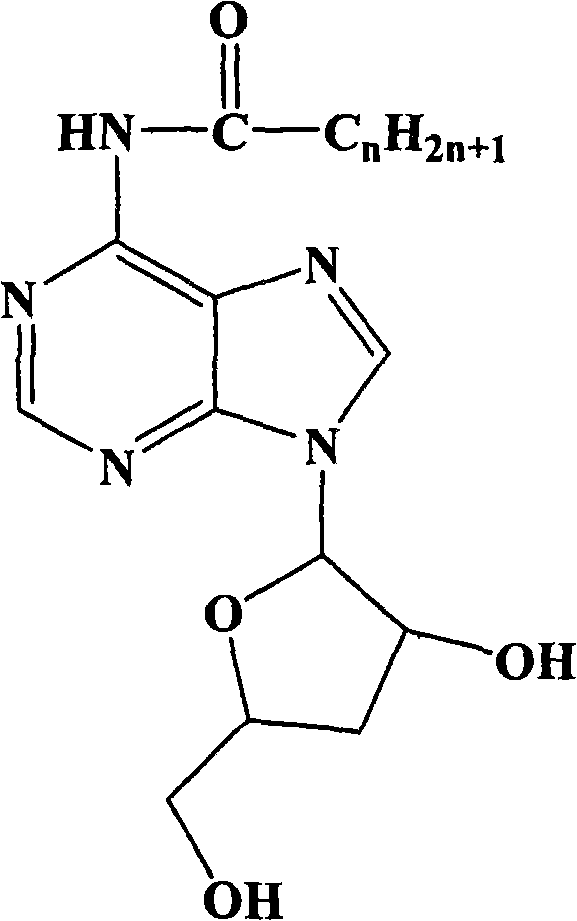
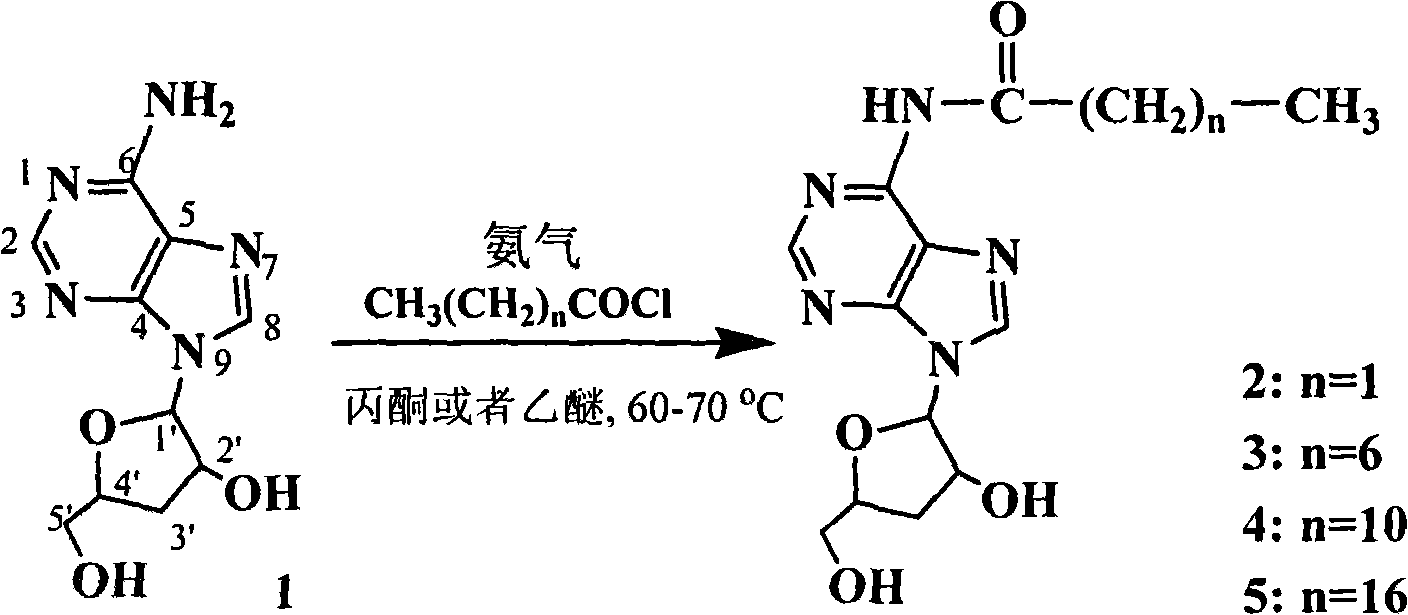
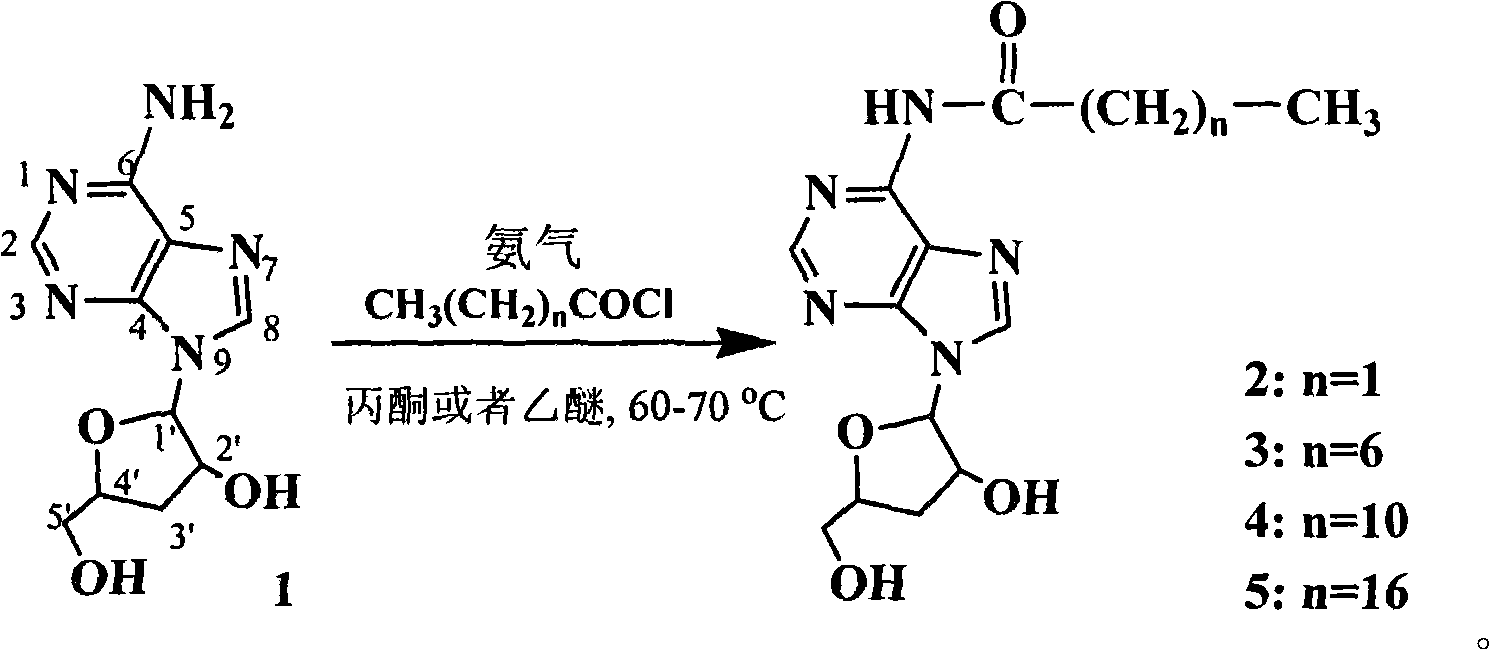
New process for synthesizing N-alkanoyl cordycepin serving as cordycepin derivative by one-step method
A synthesis process and technology of cordycepin, applied in the direction of sugar derivatives, sugar derivatives, sugar derivatives preparation, etc., can solve the problems of large pyridine pollution, unfavorable industrial development and application of compounds, low yield, etc., and achieve comprehensive cost reduction, Good application prospect, solvent cost saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] 1) Weigh 2.259g (0.009mol) of cordycepin and dissolve in 200mL of acetone (ultrasound-assisted dissolution).
[0013] 2) Under magnetic stirring and passing ammonia gas to saturation, then slowly drop 0.01 mol of alkanoyl chloride.
[0014] 3) The reaction system was incubated at 40° C. for 12 hours, and the reaction was terminated after the completion of the cordycepin reaction.
[0015] 4), after the solvent is recovered, the paste is dissolved in ethyl acetate and recrystallized to obtain the product. Yield 85%.
[0016] Synthetic process technology flow:
[0017]
[0018] Synthetic technology route
Embodiment 2
[0020] 1) Weigh 2.259g (0.009mol) of cordycepin and dissolve in 200mL of ether (ultrasound-assisted dissolution).
[0021] 2) Under magnetic stirring and passing ammonia gas to saturation, then slowly drop 0.01 mol of alkanoyl chloride.
[0022] 3) The reaction system was incubated at 80° C. for 1 hour, and the reaction was terminated after the completion of the reaction of cordycepin.
[0023] 4), after the solvent is recovered, the paste is dissolved in ethyl acetate, and the product can be obtained from crystallization. Yield 91%.
Embodiment 3
[0025] 1) Weigh 2.259g (0.009mol) of cordycepin and dissolve in 200mL of acetone (ultrasound-assisted dissolution).
[0026] 2) Under magnetic stirring and passing ammonia gas to saturation, then slowly drop 0.01 mol of alkanoyl chloride.
[0027] 3) The reaction system was incubated at 60° C. for 6 hours, and the reaction was terminated after the completion of the reaction of cordycepin.
[0028] 4), after the solvent is recovered, the paste is dissolved in ethyl acetate, and the product can be obtained from crystallization. Yield 88%.
[0029] supplementary form
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com