Application of 4-dimethyl amino nitrogen-5-hydroxy-formyl-hexyl amine for preparing anti-human immunodeficiency virus (HIV) latent medicine
A kind of technology of dimethylamino nitrogen and hexylamine, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
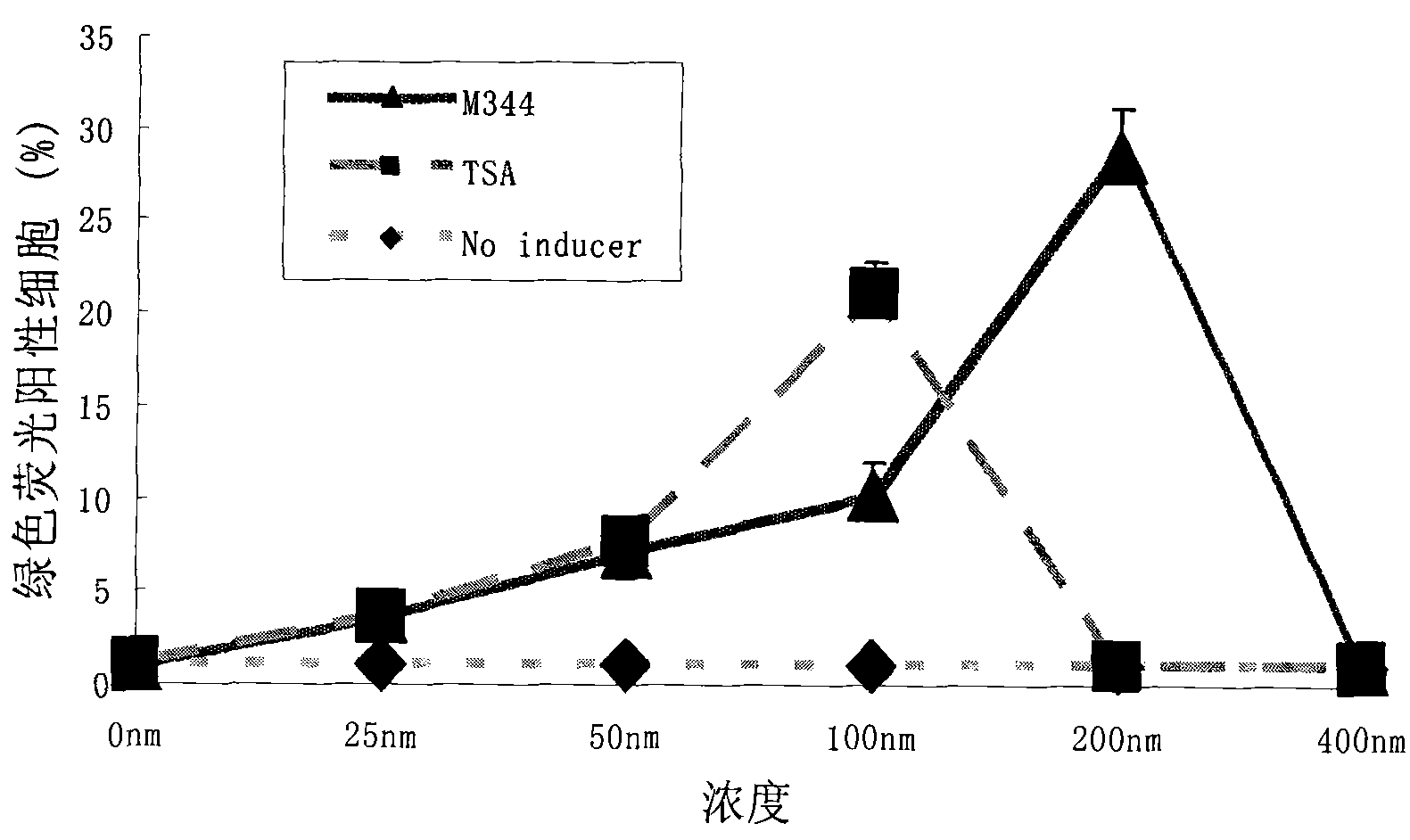
[0054] The effect concentration of embodiment 1.M344 compound is on the influence of HIV latent induction activation efficiency
[0055] A7 cells were planted in a 96-well plate at 2×10E4 cells per well, and 100 ul of 1640 medium (Gibco) containing 10% FBS (Gibco) was added to each well. After 24 hours, different concentrations of M344 were added so that the final concentrations were 0nM, 25nM, 50nM, 100nM, 200nM, 400nM. At least 3 replicate wells for each concentration, and each experiment was repeated 3 times. After the cells were treated with drugs for 72 hours, the expression of GFP in the cells was observed under a fluorescence microscope, and the cells were collected for flow cytometry detection to analyze the proportion of fluorescent cells. Treat the cells with the same concentration of the same positive control drug TSA, analyze and compare the induction and activation rate.
[0056] The results showed that with the increase of M344 compound concentration, the numbe...
Embodiment 2
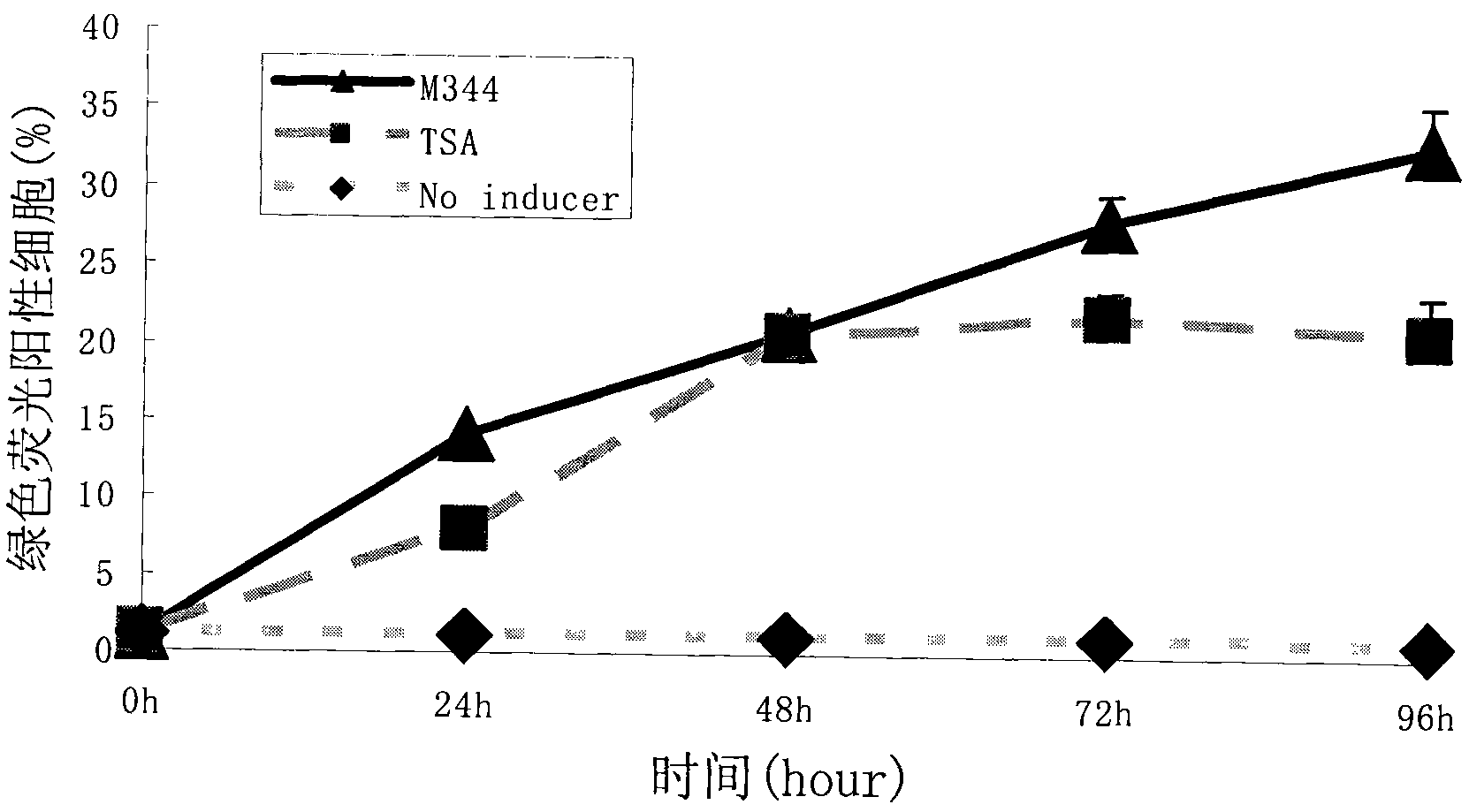
[0057] Effect of the action time of the embodiment 2.M344 compound on the efficiency of HIV latency induction and activation
[0058] 2×10E4 A7 cells per well were planted in a 96-well plate, and 100 ul of 1640 medium (Gibco) containing 10% FBS (Gibco) was added to each well. After 24 hours, M344 or TSA was added at a final concentration of 100 nM. After 24h, 48h, 72h, and 96h of drug-treated cells, the expression of GFP in the cells was observed under a fluorescent microscope, and the cells were collected for flow cytometry detection to analyze the proportion of fluorescent cells. There were at least 3 replicate wells at each time point, and each experiment was repeated 3 times. Analyze and compare the kinetic characteristics of induced activation induced activation.
[0059] The results showed that when 100nM concentrations of M344 and TSA treated HIV latent infection cell models respectively, one day later, the number of green fluorescent positive cells gradually increase...
Embodiment 3
[0060] Example 3. Toxic effect of M344 on cells
[0061] 2×10E4 normal human embryonic kidney cells (293HEK) were planted in a 96-well plate per well, and 100 ul of DMEM medium (Gibco) containing 10% FBS (Gibco) was added to each well. After 24 hours, different concentrations of M344 and TSA were added so that the final concentrations were 0nM, 25nM, 50nM, 100nM, 200nM, 400nM. At least 3 replicate wells for each concentration, and each experiment was repeated 3 times. After the cells were treated with drugs for 72 hours, MTT reagent (0.5 mg / mL) (purchased from SIGMA) was added to each well, shaken for 1 hour, and the OD value was measured at 570 nm on a microplate reader. Calculate CC50=(OD value of experimental group / OD value of control group)×100%.
[0062] The results showed that the median toxic concentration of M344 drug to normal human cells was CC50=352nM; the median toxic concentration of TSA drug to cells was CC50=170nM; the higher the CC50, the lower the cytotoxici...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com