Method for synthetizing 2,6-dimethylnaphthalene with methanol, C10 arene and 2-methylnaphthalene through alkylation
A technology for methyl decalinyl and carbon ten aromatic hydrocarbons, which is applied in chemical instruments and methods, condensation between hydrocarbons and non-hydrocarbons to produce hydrocarbons, organic chemistry, etc., and can solve serious side reactions, low synthesis yields, and product yields. Low problems, to achieve the effect of improving reactivity and rich sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
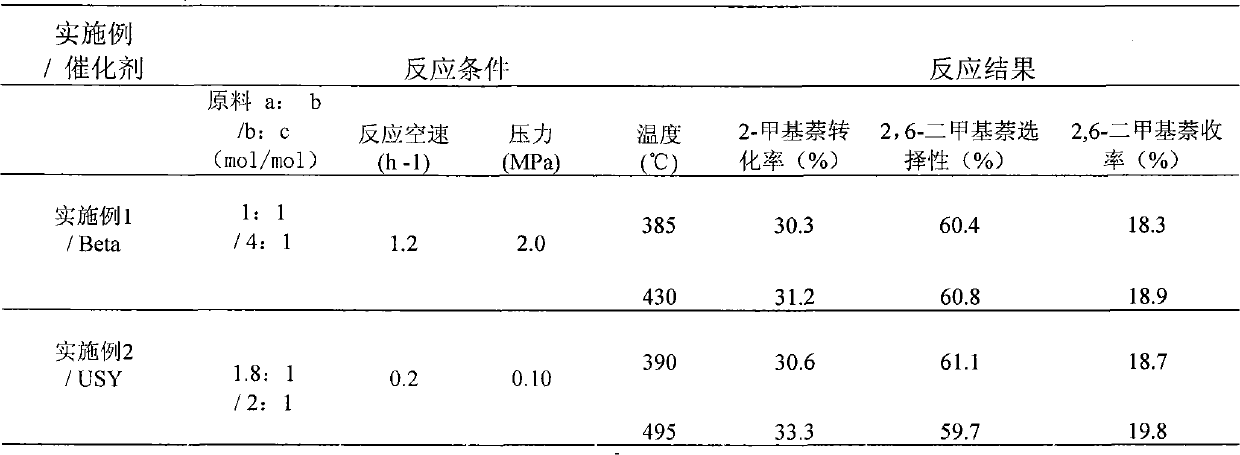
[0018] Embodiment 1: earlier the reaction tube bed bottom of the small-sized fixed-bed catalytic reactor of internal diameter 1.5cm is loaded with appropriate glass beads, spreads a thin layer of quartz wool above the glass beads, and then 5.0g cylindrical silicon-aluminum ratio is The Beta molecular sieve catalyst of 24 is loaded into the reaction tube, and the upper layer is filled with an appropriate amount of glass beads, and then the carrier gas hydrogen is introduced to ensure that the air flow evenly flows through the catalyst bed; the reaction pressure is 2.0 MPa, and the temperature is raised to the set temperature after 100 minutes. Metering pump feeding composition is a (methanol): c (2-methylnaphthalene) = 1: 1 (mol / mol), b (carbon ten aromatics): c (2-methylnaphthalene) = 4: 1 (mol / mol) of the reaction raw material, mass space velocity WHSV=1.2h -1 , from low temperature to high temperature to investigate the effect of catalytic reaction at different temperatures...
Embodiment 2
[0019] Example 2: The difference between this example and Example 1 is that the USY molecular sieve with a silicon-aluminum ratio of 5 is used as a catalyst, and the carrier gas hydrogen is introduced, and the carrier gas / reactant ratio is 4:1 (mol / mol); other The specific reaction conditions are shown in Table 1.
Embodiment 3
[0020] Example 3: The difference between this example and Example 1 is that the ZSM-5 molecular sieve with a silicon-aluminum ratio of 48 is used as a catalyst, and the carrier gas nitrogen is introduced, and the carrier gas / reactant ratio is 1.8:1 (mol / mol) ; See Table 1 for other specific reaction conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com