Method for preparing crude 2-hydroxy-4-methoxybenzophenone
A technology of methoxybenzophenone and dihydroxybenzophenone, which is applied in the field of preparation of ultraviolet absorbers, can solve the problems of many process steps, high production cost, and low product yield, and achieve simple process and environmental protection. Less pollution and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Put 440kg of dimethyl sulfate into the reaction kettle, then add 600kg of crude 2,4-dihydroxybenzophenone and 205kg of soda ash at a stirring speed of 82r / m, then heat and raise the temperature to 75°C for heat preservation reaction , A total of 8h from the start of feeding to the end of the reaction. After the reaction is finished, the material is washed with water at 70-80° C. until the pH value is 6.5-8.0, and the layers are statically separated, and the material in the lower layer is removed, and the final product is obtained through dehydration.
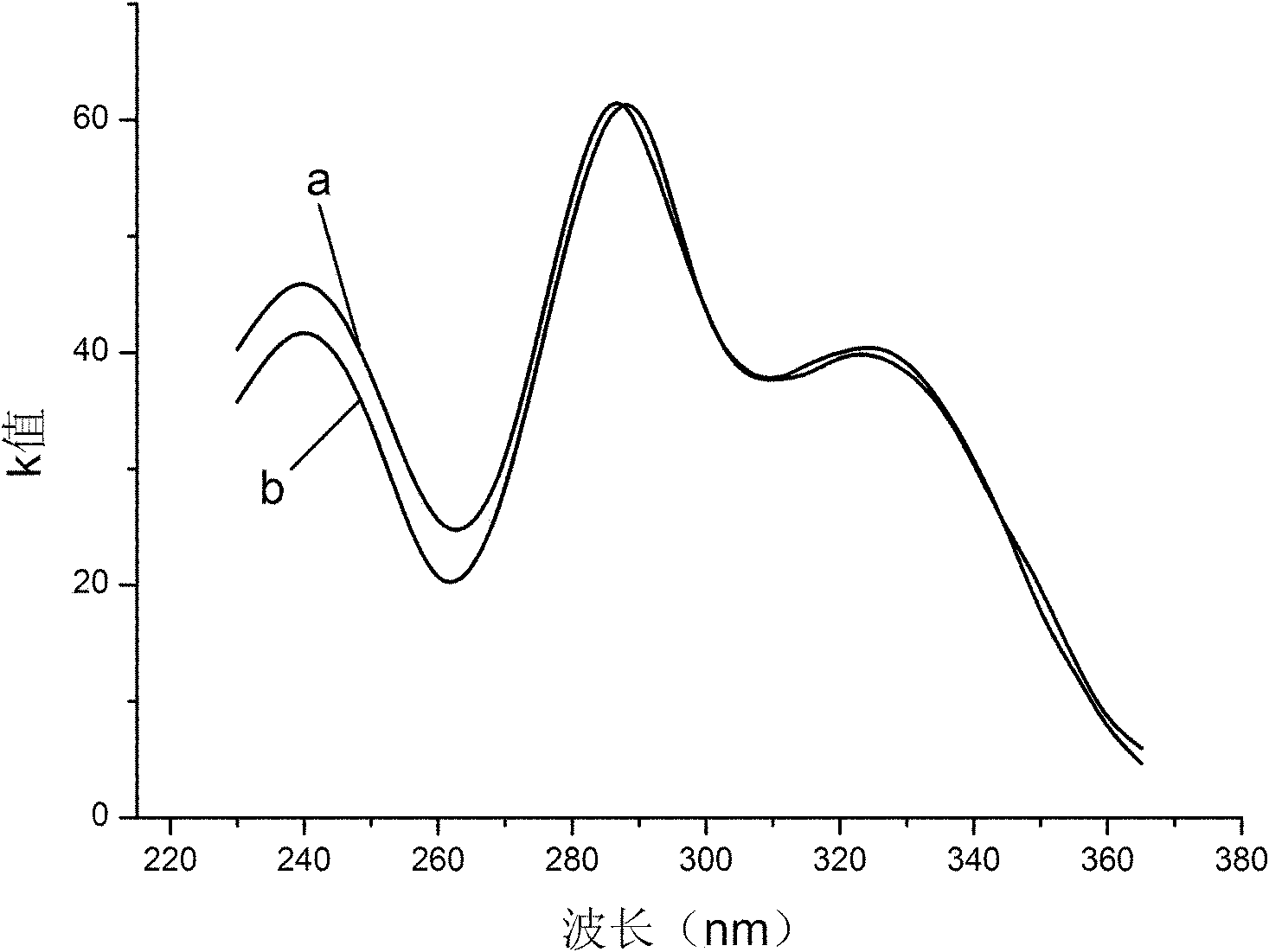
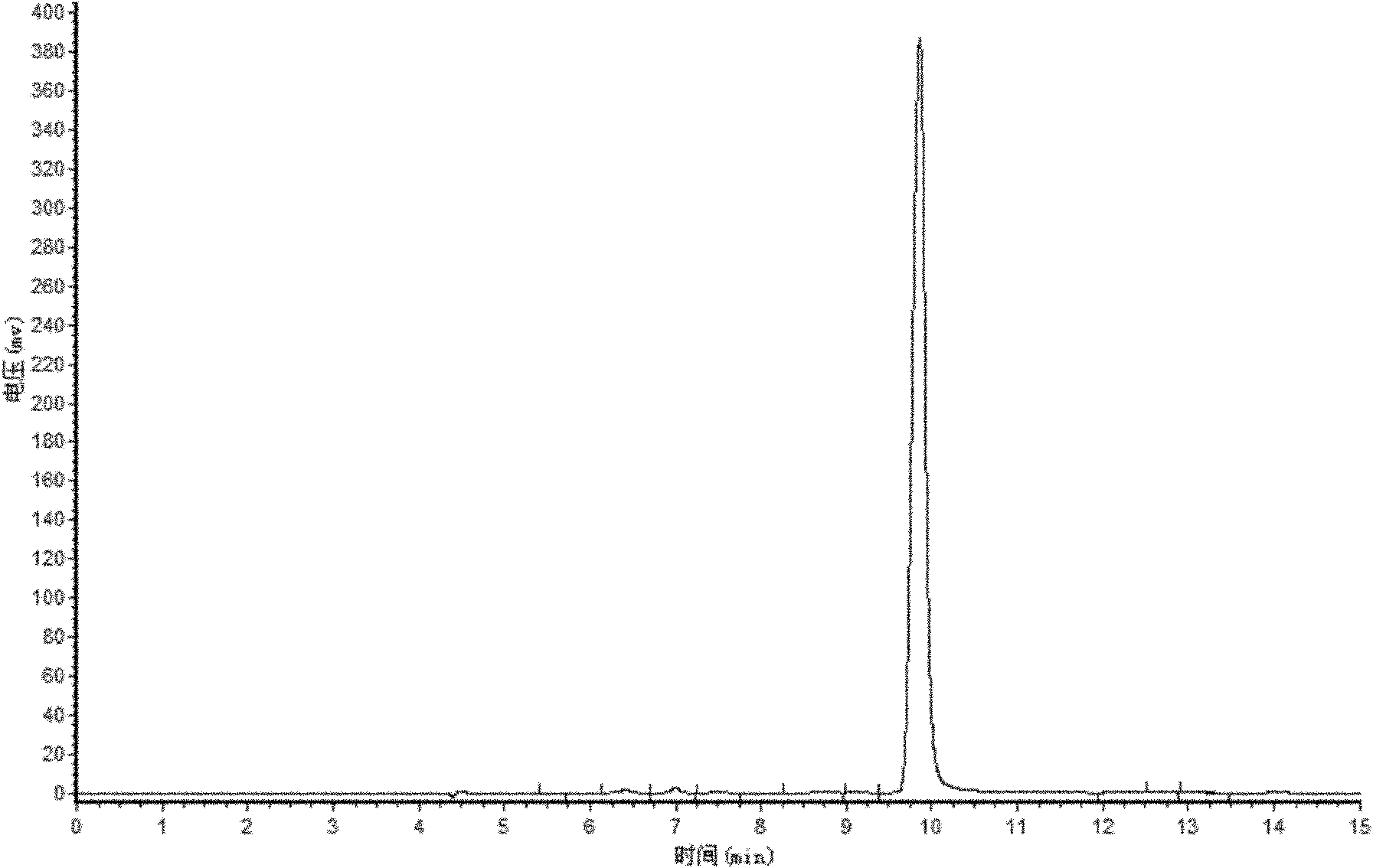
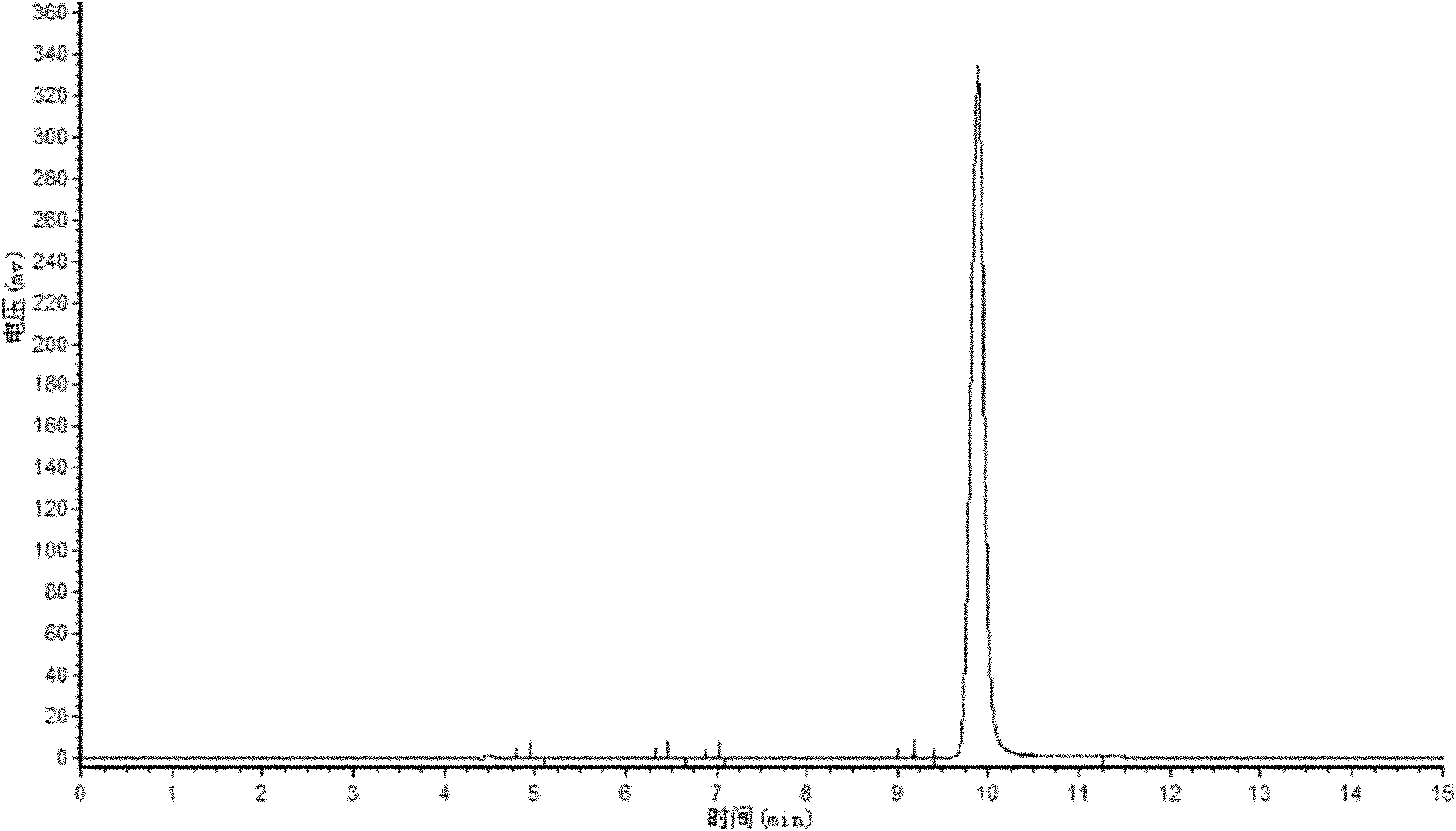
[0019] Adopt ultraviolet-visible absorption spectrometry to characterize above-mentioned product (see figure 1 Middle curve a), and the ultraviolet-visible absorption spectrum (see figure 1 In the curve b) for comparison, by figure 1 As can be seen, the absorption peaks of curve a and curve b are basically consistent; the product of this embodiment is analyzed by high performance liquid chromatography (see figure 2 ),...
Embodiment 2~3
[0021] Keep 440kg dimethyl sulfate and 600kg 2,4-dihydroxybenzophenone crude product charging amount is constant, change the charging amount of soda ash, other operating conditions and product characterization method are all the same as embodiment 1, obtain 2-hydroxyl-4 -Methoxybenzophenone. The dosage of soda ash and the test results are shown in Table 1.
[0022] Table 1. The feeding amount and test result of embodiment 2~3 soda ash
[0023]
Embodiment 4~7
[0025] Keep 600kg 2, the feed intake of 4-dihydroxybenzophenone crude product and 205kg soda ash is constant, change the feed intake of dimethyl sulfate, other operating conditions and product characterization method are all identical with embodiment 1, obtain 2-hydroxyl- 4-Methoxybenzophenone. The feeding amount and test results of dimethyl sulfate are shown in Table 2.
[0026] Table 2. The charging capacity and test result of embodiment 4~7 dimethyl sulfate
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com