Polyhydroxy compound, method for manufacturing the same and epoxy resin composition, cured product thereof
A technology of polyhydric hydroxyl and epoxy resin, which is applied in the preparation of amino hydroxyl compounds, organic compounds, and lubricating compositions. It can solve the problems of large steric barriers and fragility, and achieve excellent flame retardancy and high heat resistance. , High adhesion and excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Add 46.5g (0.5 mol) of aniline and 200g (1.0 mol) of 4,4'-dihydroxydiphenylmethane, heat to 80°C while introducing nitrogen, and drop 40.5g (0.5 mol) of 37% formalin aqueous solution . Next, the temperature was raised to 95° C. and refluxed for 2 hours while stirring, and after further dehydration, the temperature was raised to 180° C. and reacted for 2 hours. Thereafter, unreacted aniline was removed under reduced pressure at 180° C. to obtain 238.5 g of an amino group-containing resin. The hydroxyl equivalent of the obtained resin was 117.4 g / eq., the amine equivalent was 626.4 g / eq., the softening point was 62° C., and the melt viscosity at 150° C. was 31 mPa·s.
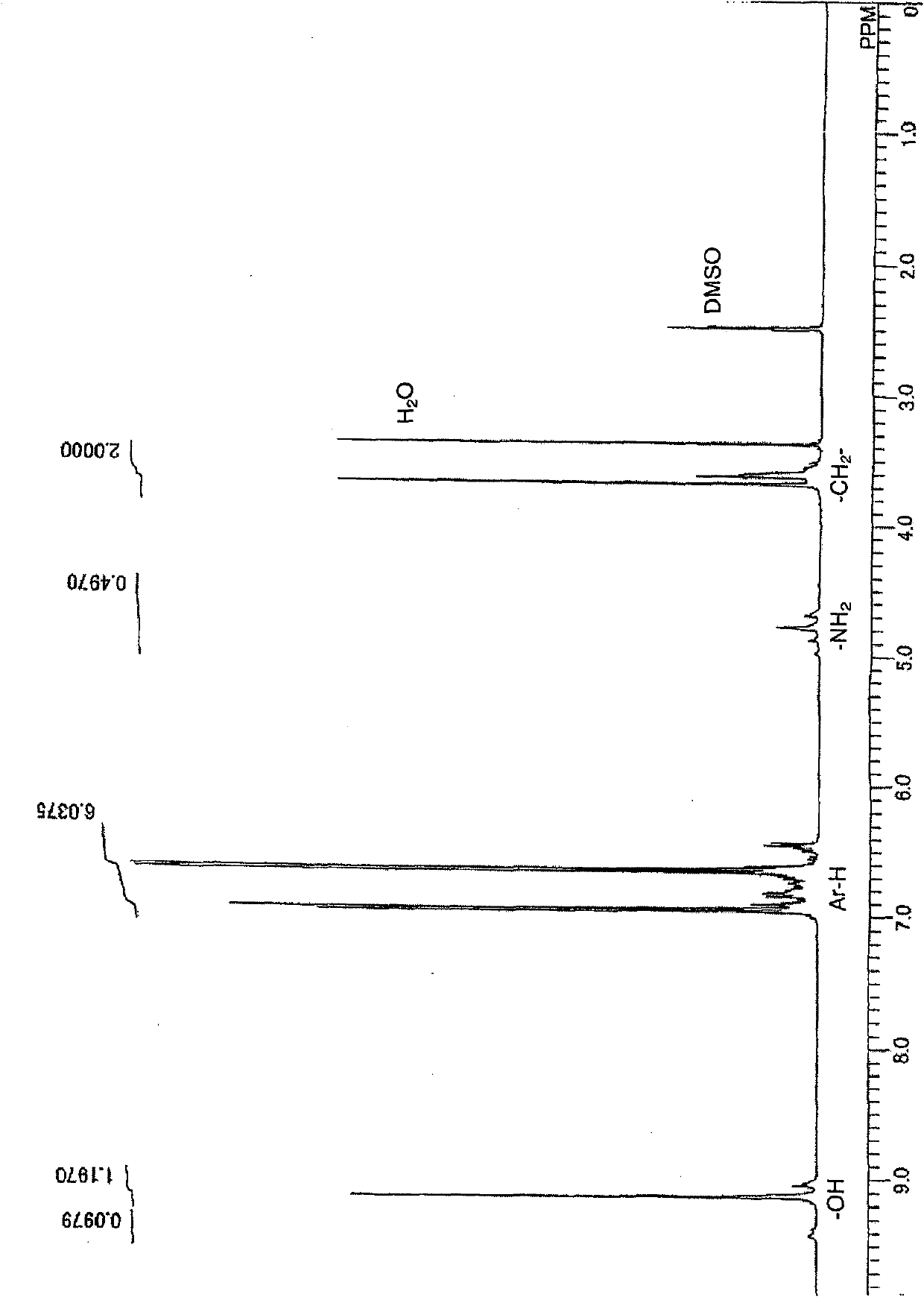
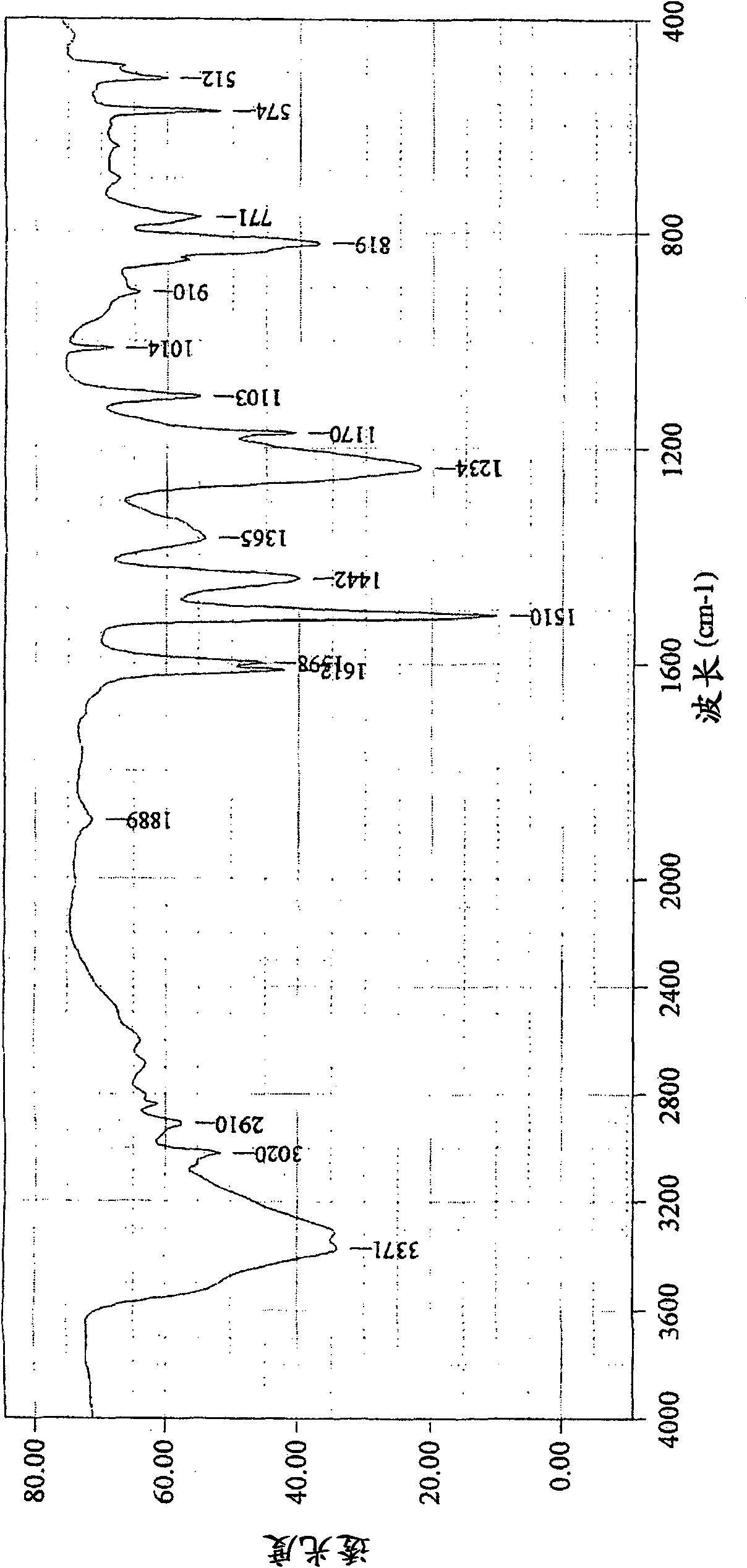
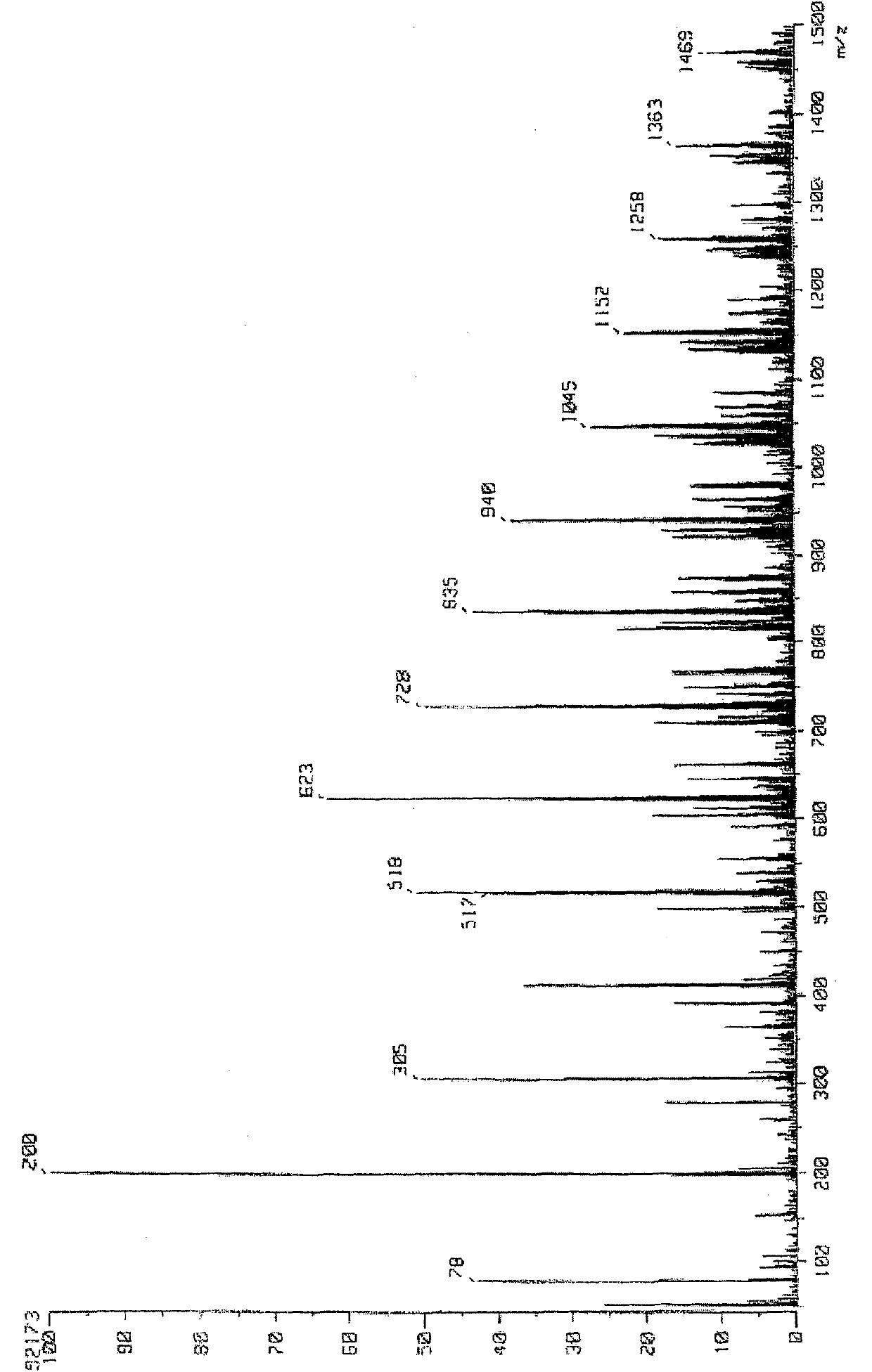
[0109] 1 The H-NMR spectrum is shown in figure 1 , the infrared absorption spectrum is shown in figure 2 , the FDMS spectrum is shown in image 3 , the GPC spectrum is shown in Figure 4 . In the FDMS spectrogram, it can be observed that m / z=200 corresponding to n=1 and m=0 in formula (1), m / z=305 c...
Embodiment 2
[0111] 60 g of the amino group-containing resin obtained in Example 1 and 14.2 g of phthalic anhydride were charged, and heated to 150° C. to dissolve and react for 1 hour while introducing nitrogen. Meanwhile, the water generated by the reaction was excluded from the reaction system. Thereafter, unreacted phthalic anhydride was removed under reduced pressure at 230° C. to obtain 57.1 g of imino group-containing resin (resin A). The obtained resin had a hydroxyl equivalent of 157 g / eq., an amine equivalent of 74950 g / eq., a softening point of 76°C, and a melt viscosity of 0.11 Pa·s at 150°C.
[0112] 1 The H-NMR spectrum is shown in Figure 5 , the infrared absorption spectrum is shown in Figure 6 , the FDMS spectrum is shown in Figure 7 , the GPC spectrum is shown in Figure 8 . In the FDMS spectrogram, it can be observed that m / z=200 corresponding to n=1 and m=0 in formula (5), m / z=435 corresponding to n=1 and m=1, corresponding to n=2 And m / z=647 of m=1, m / z=670 co...
Embodiment 3
[0114] Add 60 g of the amino group-containing resin obtained in Example 1, 9.4 g of maleic anhydride, and 120 g of toluene, heat to 110° C. while introducing nitrogen, and react for 2 hours while removing water from the reaction system by azeotroping with toluene. Thereafter, it was heated to 180° C. under reduced pressure to remove toluene and unreacted maleic anhydride to obtain 64.3 g of an imino group-containing resin (resin B). The hydroxyl equivalent of the obtained resin was 144 g / eq., the amine equivalent was 138400 g / eq., the softening point was 88.5°C, and the melt viscosity at 150°C was 0.44 Pa·s. The GPC spectrum is shown in Figure 9 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com