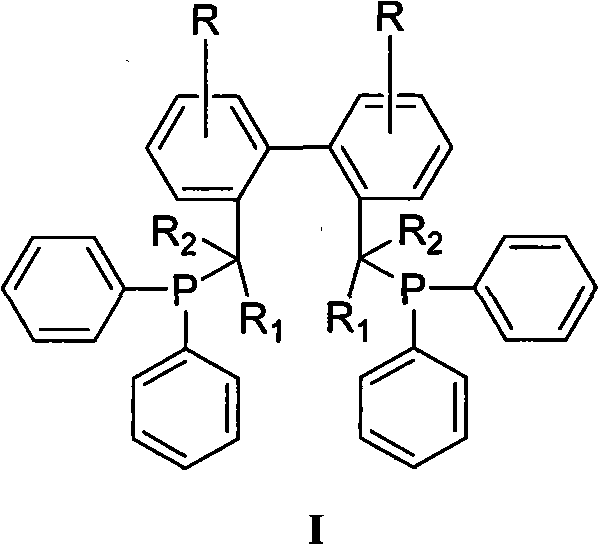
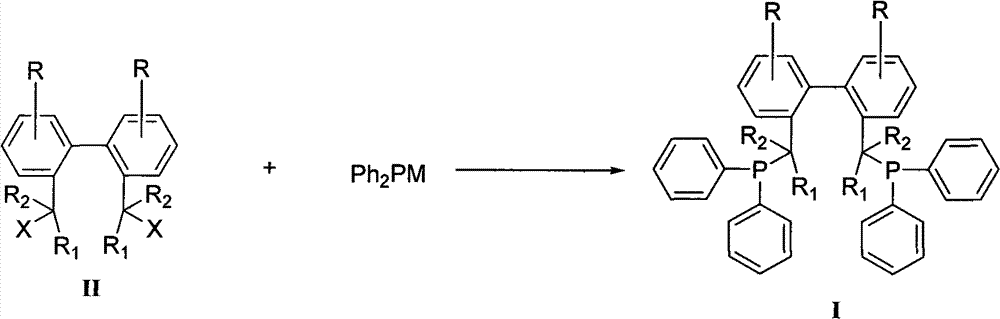
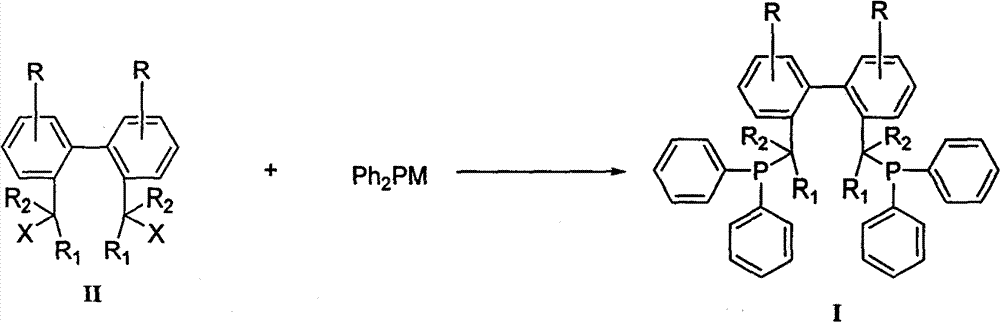
Method for preparing biphenyl diphosphine ligand
A technology of diphosphine ligand and biphenyl, which is applied in the field of synthesis of organic compounds, can solve the problems of cumbersome post-treatment, low yield, oxidation of phosphine ligand, etc., and achieve the effect of simple operation and cheap and easy-to-obtain reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 50 parts of triphenylphosphine and 260 parts of tetrahydrofuran into the dry reaction bottle under the protection of nitrogen, stir and dissolve, add 14 parts of metal sodium, heat, stir and reflux for 12 hours. Cool to 0°C, add 15 parts of tert-butyl chloride dropwise, heat the reaction solution under reflux for 0.5h after addition, then cool to 0°C again, add dropwise 2,2'-dibromomethyl-1,1'- Biphenyl II (R = R 1 = R 2 =-H, X=Br) 20 parts and 20 parts of tetrahydrofuran in a mixed solution, add to room temperature and stir for 12h. The reaction solution was extracted, dried, and concentrated to obtain a crude product, which was recrystallized from absolute ethanol to obtain a white solid (BISBI), with a yield of 78%, Mp: 83.2-85.4°C.
[0027] 1 H NMR (CDCl 3 , 400MHz) δ7.53~7.00 (m, 28H), 3.26~3.02 (m, 4H).
[0028] 31 P NMR (CDCl 3 , 161.7MHz) δ-10.56(s).
Embodiment 2
[0030] Add 60 parts of triphenylphosphine and 290 parts of tetrahydrofuran into a dry reaction flask under the protection of nitrogen, stir and dissolve, add 20 parts of sodium metal, and 3.0 parts of naphthalene, heat and stir to reflux for 2 hours. Cool to 0°C, add 19 parts of tert-butyl chloride dropwise, heat the reaction solution under reflux for 0.5h after addition, then cool to 0°C again, add dropwise 2,2′-dimethylsulfonate-1,1 '-biphenyl II (R = R 1 = R 2 =-H, X=-CH 2 OSO 2 CH 3 ) 30 parts and THF 30 parts of the mixed solution, after adding, it was raised to room temperature and stirred for 12h. The reaction solution was extracted, dried, and concentrated to obtain a crude product, which was recrystallized from isopropanol to obtain a white solid (BISBI), with a yield of 80%, Mp: 84.2-86.2°C.
[0031] 1 H NMR and 31 The P NMR spectrum is consistent with Example 1.
Embodiment 3
[0033] Add 50 parts of triphenylphosphine and 295 parts of 2-methyltetrahydrofuran into the dry reaction bottle under the protection of nitrogen, stir and dissolve, add 3.3 parts of metal lithium, and stir at room temperature for 12 hours. Cool to 0°C, add 17 parts of tert-butyl chloride dropwise, heat the reaction solution under reflux for 0.5h after addition, then cool to 0°C again, add dropwise 2,2′-di-p-toluenesulfonate methyl-1, 1'-biphenyl II (R=R 1 = R 2 =-H, X=-CH 2 OSO 2 PhCH 3 -p) A mixed solution consisting of 40 parts of 2-methyltetrahydrofuran and 40 parts of 2-methyltetrahydrofuran was added and raised to room temperature and stirred for 6 hours. The reaction solution was extracted, dried, and concentrated to obtain a crude product, which was recrystallized from acetone to obtain a white solid (BISBI), with a yield of 75%, Mp: 83.8-86.1°C.
[0034] 1 H NMR and 31 The P NMR spectrum is consistent with Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com