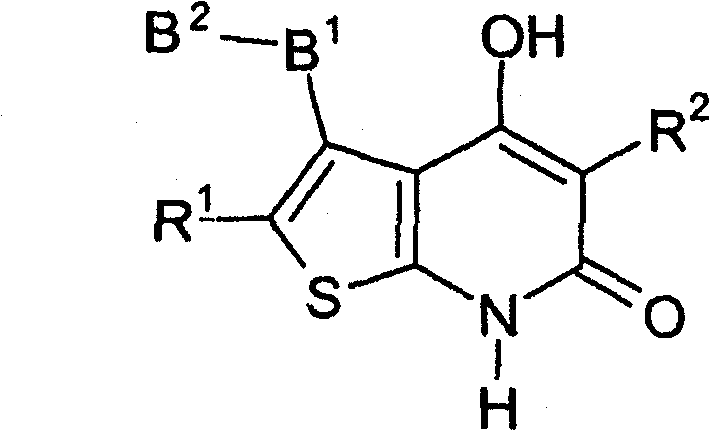
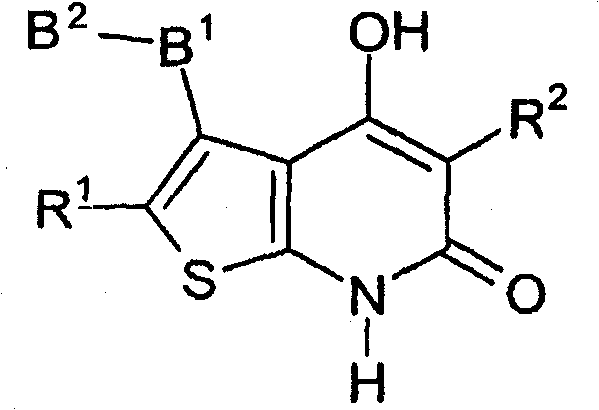
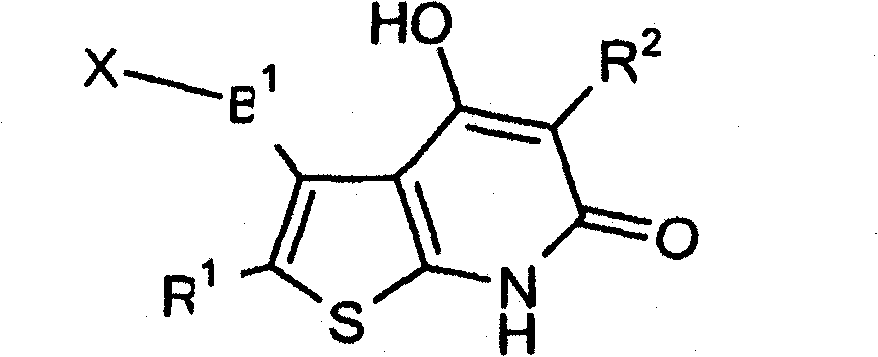
Thienopyridone derivatives as AMP-activated protein kinase (AMPK) activators
A pyridine, thiophene technology, used in obesity, treatment of diseases such as diabetes, protein kinase activator, use in inflammation, metabolic syndrome, preparation of thienopyridone, cancer field, can solve problems such as energy expenditure and weight loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0303] 2-Chloro-4-hydroxy-3-(2′-methoxybiphenyl-4-yl)-5-phenyl-6,7-dihydro-thieno[2,3-b]pyridine-6- ketone
[0304] Step 1: Intermediate 1 Step 1 (2g, 4.50mmol), 2-methoxyphenylboronic acid (1.37g), cesium carbonate (4.40g) and tetrakis(triphenylphosphine)palladium (468mg) were mixed under argon ) in a mixture of toluene (55 mL) / ethanol (65 mL) / water (32 mL) was heated at 80° C. overnight. Filter the solution through Pad filter and dissolve in ethyl acetate. The organic solution was washed with hydrochloric acid solution (4M), then dried over sodium sulfate. The solvent was removed under reduced pressure and the resulting crude solid (1.57 g) was washed with a mixture of petroleum ether / minimum ethyl acetate.
[0305] 1 H NMR (DMSO-d 6 , 300MHz) δ[ppm] 11.01(bs, 1H), 7.67-7.03(m, 13H), 6.99(s, 1H), 4.08(q, 2H), 3.96(s, 2H), 3.78(s, 3H) , 0.96(t,3H).
[0306] Step 2: A solution of the above compound (1.54 g, 3.27 mmol), N-chlorosuccinimide (0.48 g) in chloroform (20 mL...
Embodiment 2
[0312] 4-Hydroxy-3-(2'-hydroxybiphenyl-4-yl)-5-phenyl-6,7-dihydro-thieno[2,3-b]pyridin-6-one
[0313] Intermediate 1 (0.4 g, 1 mmol), 2-hydroxyphenylboronic acid (277 mg), cesium carbonate (981 mg) and tetrakis(triphenylphosphine)palladium (100 mg) were dissolved in dimethylformamide (10 mL) under argon. ) / toluene (1 mL) / ethanol (6 mL) / water (3 ml) mixture was heated at 80° C. overnight. Filter the solution through Pad, and concentrated under reduced pressure. Add acetonitrile. The precipitated solid (244 mg) was filtered and washed by water, hydrochloric acid solution (4M), acetonitrile and petroleum ether;
[0314] MS: 412.1(M+1);
[0315] 1 H NMR (DMSO-d 6 , 300MHz) δ [ppm] 7.52-7.45 (m, 4H), 7.37-7.23 (m, 6H), 7.17-7.11 (m, 1H), 7.04 (s, 1H), 6.96-6.84 (m, 2H).
Embodiment 3
[0317] 4-Hydroxy-3-(2'-hydroxybiphenyl-4-yl)-5-(pyridin-3-yl)-6,7-dihydro-thieno[2,3-b]pyridin-6-one
[0318] Intermediate 2 (1 g, 2.50 mmol), 2-hydroxyphenylboronic acid (0.69 g), cesium carbonate (2.45 g) and tetrakis(triphenylphosphine)palladium (260 mg) were dissolved in dimethylformamide under argon. A solution in a mixture of (25 mL) / benzene (2.6 mL) / ethanol (15 mL) / water (7.5 mL) was heated at 80° C. for 6 hours. pass the solution through Pad filtered and concentrated under reduced pressure. Add acetonitrile. The precipitated solid (629 mg) was filtered and washed with water, acetone, ethyl acetate and petroleum ether;
[0319] MS: 413(M+1);
[0320] 1H NMR (DMSO-d 6 , 300MHz) δ [ppm] 9.66 (bs, 1H), 8.90 (m, 1H), 8.79-8.77 (m, 1H), 8.56-8.53 (m, 1H), 8.06-8.01 (m, 1H), 7.54- 7.47 (m, 4H), 7.26-7.09 (m, 3H), 7.00-6.83 (m, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com