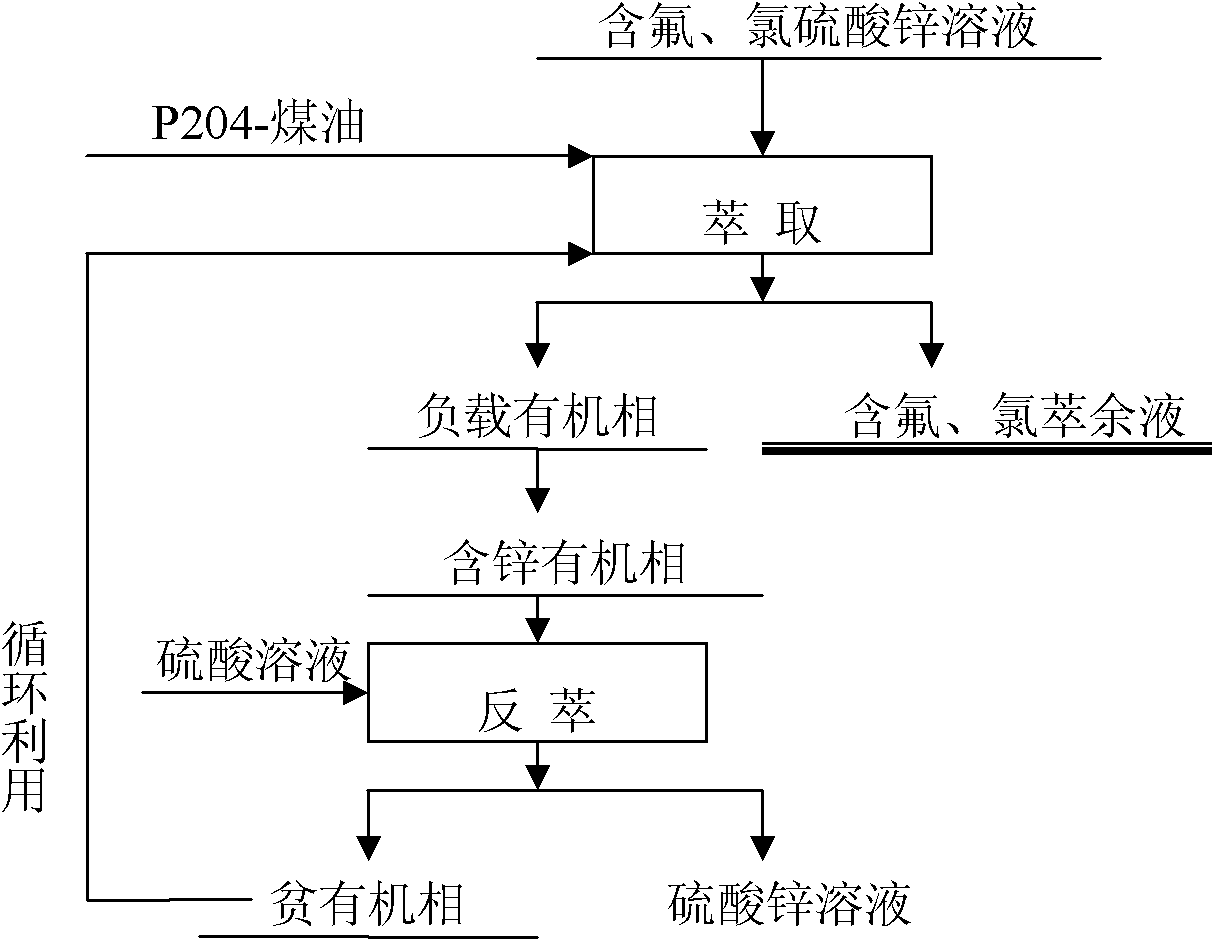
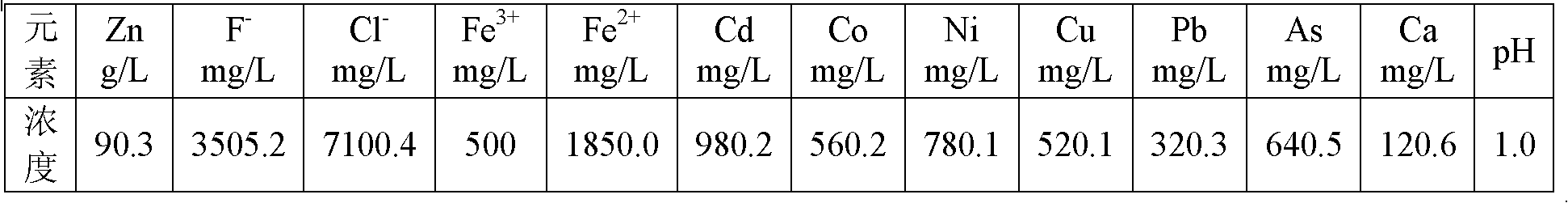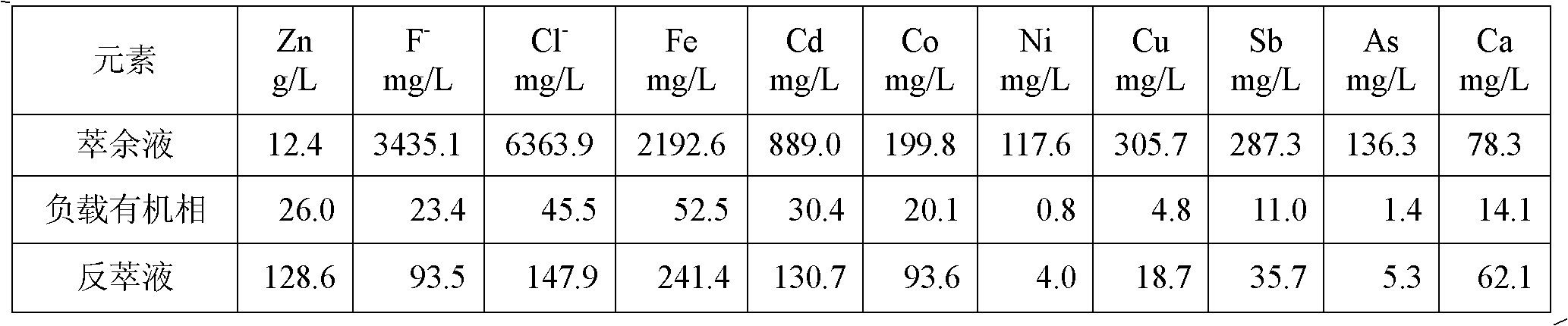Method for separating zinc, fluorine and chlorine from fluorine- and chlorine-containing zinc sulfate solution
A technology of zinc chlorosulfate and solution, applied in the field of separation of zinc, fluorine and chlorine, can solve the problems of poor fluorine removal effect, difficult process control, limited removal efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The zinc sulfate solution obtained by volatilizing the secondary zinc oxide powder obtained by using the rotary kiln and leaching with sulfuric acid, the chemical composition of the solution is listed in the following table:
[0020]
[0021] Due to the high concentration of Fe(III) and Cd ions in the solution, Fe(III) is easier to be extracted by P204 than zinc, and it is difficult to strip, but Fe(II) is difficult to be extracted by P204. Part of Cd is extracted into the organic phase and is easy to back-extract. Therefore, it is necessary to add zinc powder to reduce the easily extracted Fe(III) to difficultly extracted Fe(II), and replace cadmium with metal cadmium to remove. When the addition of Zn powder is 5g / L, the reaction time is 30min, the temperature is 20°C, the reduction rate of Fe(III) is 97.3%, and the removal rate of cadmium is 98.7%. Calcium oxide was added to adjust the pH of the solution to 4.5, and 6-stage countercurrent extraction was carried o...
Embodiment 2
[0025] The zinc sulfate solution leached by sulfuric acid from blast furnace zinc-containing dust is used, and the chemical composition of the solution is listed in the following table:
[0026]
[0027] The addition of Zn powder is 5g / L, the reaction time is 30min, the temperature is 25°C, the reduction rate of Fe(III) is 98.7%, and the removal rate of cadmium is 99.6%. Add calcium oxide to adjust the pH of the solution to 4.0, carry out 5-stage countercurrent extraction, compare (O / A) 2:1, mixing time 2-3min, clarifying time 5min, organic phase composition 30% P204+70% sulfonated kerosene, extraction The temperature is 25°C. During the extraction process, lye needs to be supplemented to maintain the pH of the raffinate at 2.0-3.5. The zinc extraction rate is 87.4%. The loaded organic phase contains about 17.7g / L of zinc, and fluorine and chlorine are not extracted. Stripping agent uses 180g / L sulfuric acid solution. The stripping mixing time is 2 minutes, the ratio (O / A)...
Embodiment 3
[0031] Adopt Zn-MnO Waste dry cell through the zinc sulfate solution of sulfuric acid leaching, the chemical composition of solution is listed in the following table:
[0032]
[0033]When the added amount of Zn powder is 3g / L, the reaction time is 30min, the temperature is 25°C, the reduction rate of Fe(III) is 98.3%, and the removal rate of cadmium is 99.4%. Calcium oxide was added to adjust the pH of the solution to 5.0, and 4-stage countercurrent extraction was carried out. The ratio (O / A) was 1:1, the mixing time was 2-3 minutes, and the clarification time was 5 minutes. The organic phase composition was 25% P204+80% sulfonated kerosene, and the extraction The temperature is 25°C, lye needs to be added during the extraction process to maintain the pH of the raffinate at 2.0-3.5, the zinc extraction rate is 88.3%, the loaded organic phase contains about 16.3g / L of zinc, and fluorine and chlorine are not extracted. Stripping agent uses 150g / L sulfuric acid solution. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com