Cycloastragenol-6-O-beta-D glucoside monohydrate and crystal thereof
A technology of cycloastragenol and glucoside, which is applied in the field of medicine and can solve the problems of easy moisture absorption, difficult storage, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
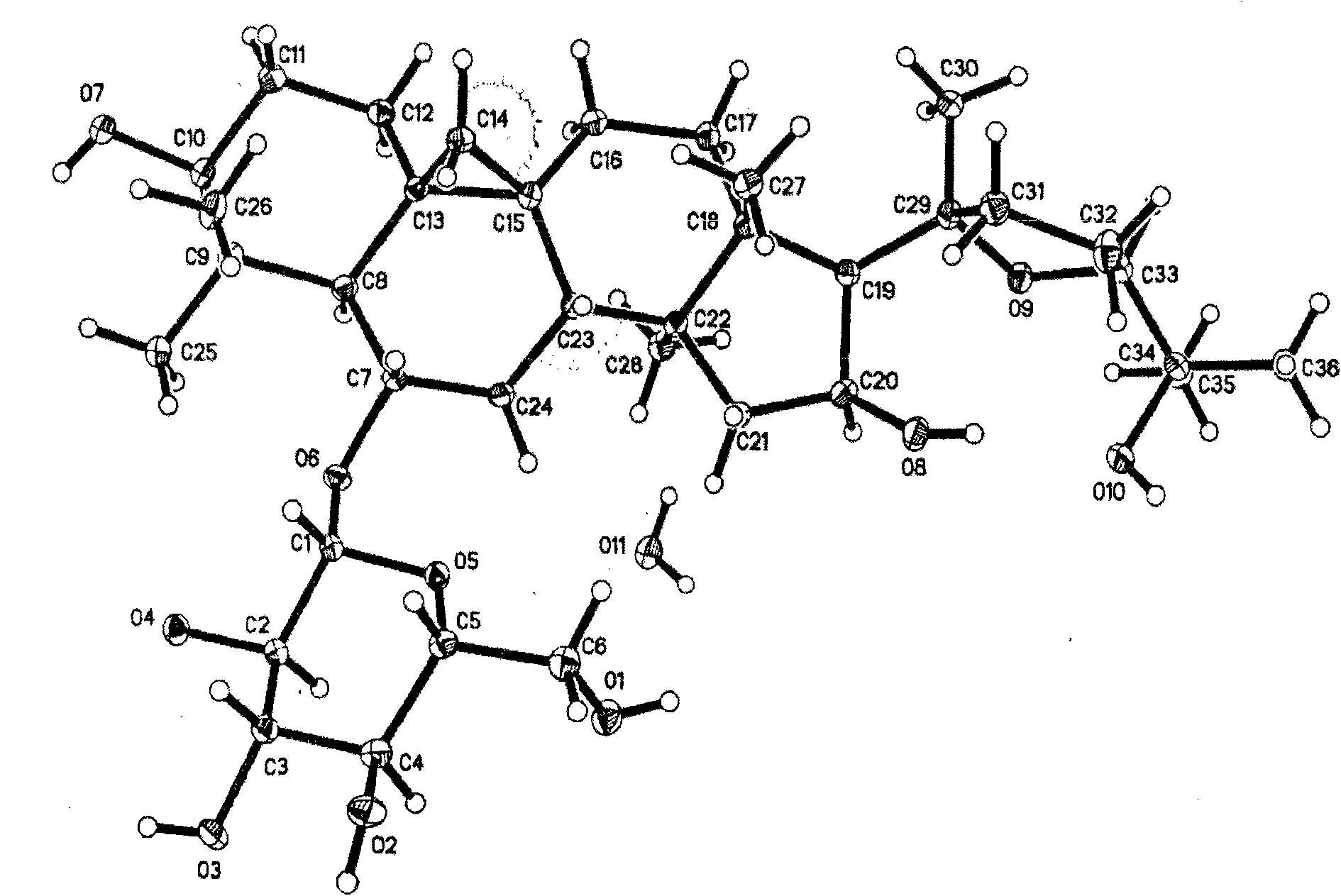
[0039] CMG 5g, heated and dissolved with 50ml 70% (volume) ethanol aqueous solution, filtered while hot, added ethyl acetate to the filtrate under stirring until the volume ratio of ethyl acetate to ethanol in the solution was 10:1, left at room temperature for 24 hours, and white needles were precipitated. crystallized, filtered. Vacuum dry at 105°C for 24 hours, and dry in an infrared dryer for 1 hour to obtain a CMG monohydrate type I single crystal, whose diffraction three-dimensional structure projection diagram is shown in figure 1 ,
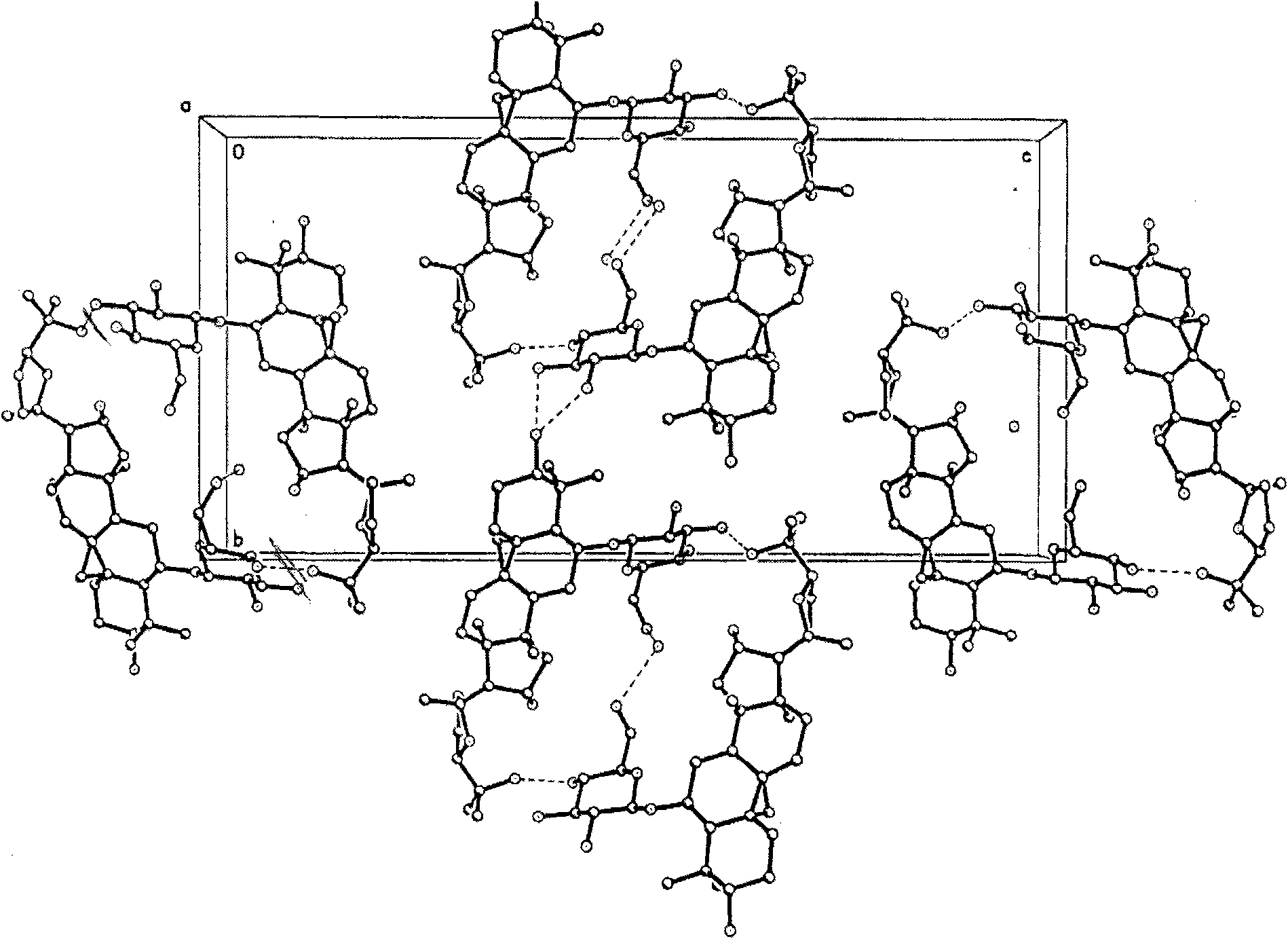
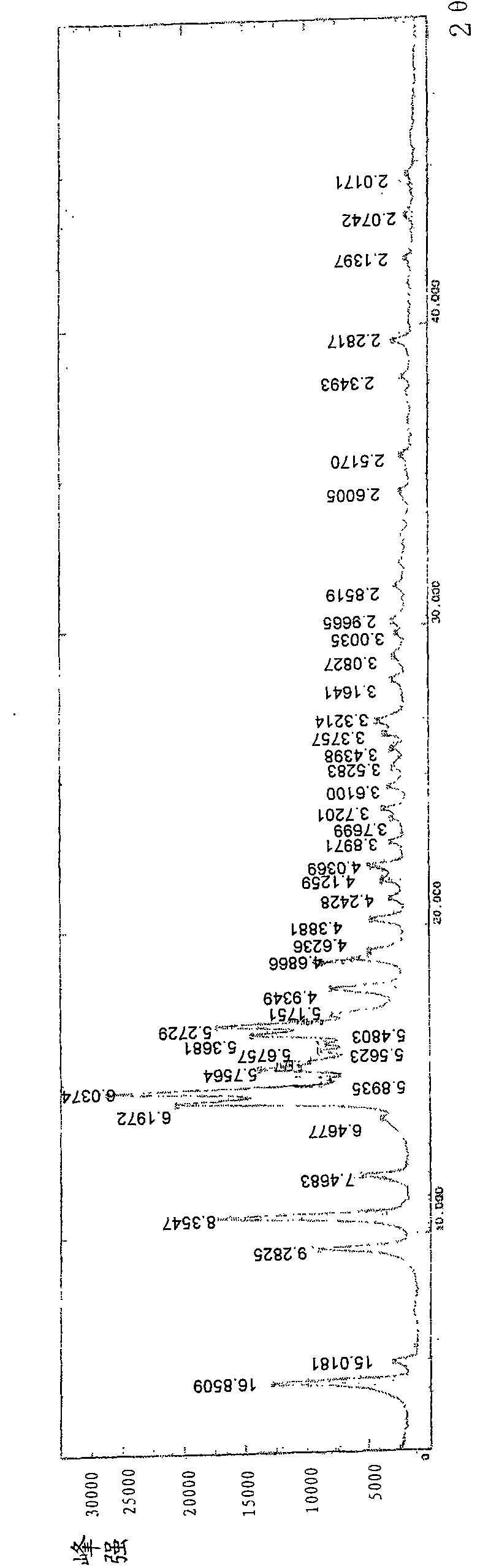
[0040] The projection diagram of the molecules in the single crystal diffraction unit cell along the α-axis direction is shown in figure 2 . The single crystal is ground into powder, and the powder diffraction of CMG monohydrate type I crystal is carried out. The X-ray powder diffraction pattern represented by 2θ angle is shown in image 3 , Table 1 shows the X-ray single crystal diffraction atomic coordinate parameters and equivalent ...
Embodiment 2
[0057] CMG 5g, heated and dissolved with 100ml 80% (volume) ethanol aqueous solution, filtered while it was hot, added ethyl acetate to the filtrate under stirring until the volume ratio of ethyl acetate to ethanol in the solution was 8:1, left at room temperature for 36 hours, and precipitated white Needle crystal, filtered. Vacuum drying at 60°C for 12 hours gave type I CMG monohydrate crystals. Thermogravimetric analysis showed a weight loss of 3.1% at 70-120°C.
Embodiment 3
[0059] CMG 5g, heated and dissolved with 60ml 90% (volume) ethanol aqueous solution, filtered while hot, added ethyl acetate to the filtrate under stirring until the volume ratio of ethyl acetate to ethanol in the solution was 6:1, left at room temperature for 48 hours, and precipitated white Needle crystal, filtered. Dry at room temperature for 48 hours to obtain type I CMG monohydrate crystals. Thermogravimetric analysis showed a weight loss of 2.7% at 70-120°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com