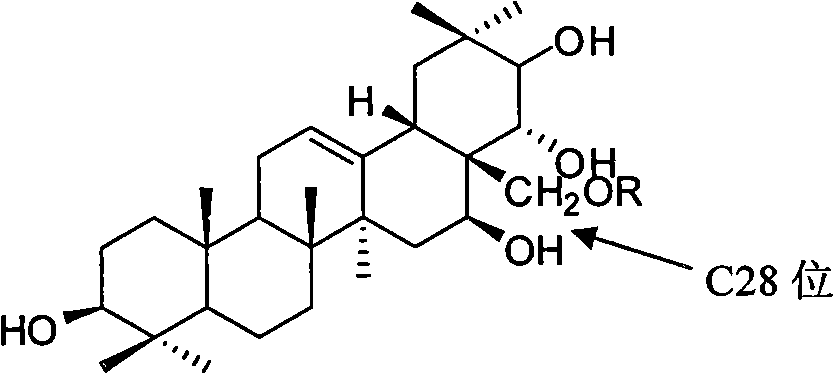
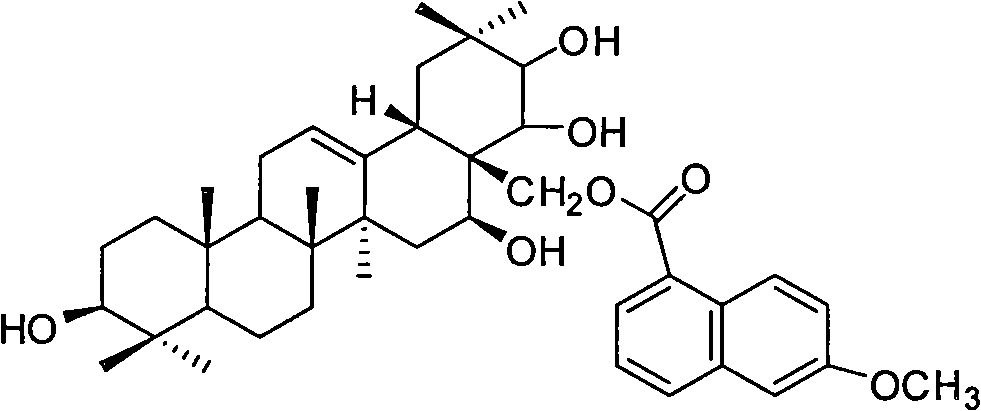
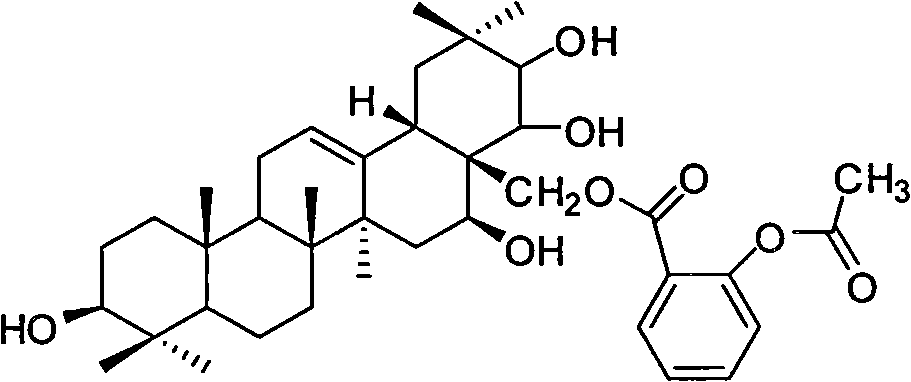
Tea saponin derivative with anti-inflammatory analgesic efficacy, preparation method and application thereof
A tea saponin, anti-inflammatory and analgesic technology, which is applied in the field of medicine, can solve the problems of low analgesic potency and affect clinical application, and achieve the effects of easy control of reaction conditions, guaranteed consistency, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Theosapogenin (C 30 h 50 o 5 ) solid 100g, added to 300mL of pyridine, heated to 50°C to dissolve, added triphenylchloromethane (56.7g) according to the equimolar amount of tea saponin, kept stirring at 50°C for 8h; added triphenylchloromethane 1 / 5 mass of potassium hydroxide (11.3g), heated to 80°C, stirred for 1h, evaporated under reduced pressure to remove water and pyridine, added 500mL of toluene to dissolve, heated to 120°C, slowly added dropwise Benzyl bromide (140g), stirred and reacted for 8h; Naproxen (46.9g) was added in an equimolar amount of teasapogenin, and 12mL of formic acid was refluxed for 5h; The catalyst was fed with hydrogen for 3 hours, and the precipitate was crystallized with ethanol water to obtain 73 g of the product.
[0037] The structural analysis of the product shows that MS: m / z 674; the elemental composition is: C74.74%, H8.66%, O16.59%, and its molecular formula is C 42 h 58 o 7 , 1 H-NMR with 13 The hydrogen and carbon signals ...
Embodiment 2
[0040] Theosapogenin (C 30 h 50 o 5 ) solid 100g, was added to 300mL of pyridine, dissolved at room temperature (25°C), triphenylchloromethane (85g) was added according to 1.5 times the molar amount of tea saponin, and stirred for 16h; triphenylchloromethane was added for 1 / 5 mass of potassium hydroxide (17g), heat up to 80°C, stir for 1h, evaporate water and pyridine under reduced pressure, add 500mL of toluene to dissolve, heat up to 140°C, slowly add benzyl bromide with 5 times the molar mass of tea saponin (174g), stirring reaction 6h; Add the naproxen (46.9g) of tea saponin equimolar amount, formic acid 18mL reflux divides water 3h; Reactant is placed in pressure reactor, with 10% palladium carbon as catalyst, Hydrogen was introduced for 3 hours, and the precipitate was crystallized with ethanol water to obtain 84 g of the product.
[0041] The analysis data of the product structure is the same as that in Example 1, which proves that the final product is naproxenate t...
Embodiment 3
[0043] Theosapogenin (C 30 h 50 o 5) solid 100g, added to 300mL of pyridine, heated to 40°C to dissolve, added triphenylchloromethane (68g) according to 1.2 times the molar mass of tea saponin, kept stirring at 40°C for 12h; added triphenylchloromethane 1 / 5 mass of potassium hydroxide (13.6g), heated to 80°C, stirred for 1h, evaporated under reduced pressure to remove water and pyridine, added 500mL of toluene to dissolve, heated to 130°C, slowly added dropwise 4.5 times the molar amount of tea saponin Benzyl bromide (157g), stirred and reacted for 7h; Naproxen (46.9g) was added in an equimolar amount of teasapogenin, and 15mL of formic acid was refluxed for water separation for 4h; The catalyst was fed with hydrogen for 3 hours, and the precipitate was crystallized with ethanol water to obtain 68 g of the product.
[0044] The analysis data of the product structure is the same as that in Example 1, which proves that the final product is naproxenate theasapogenol ester.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com