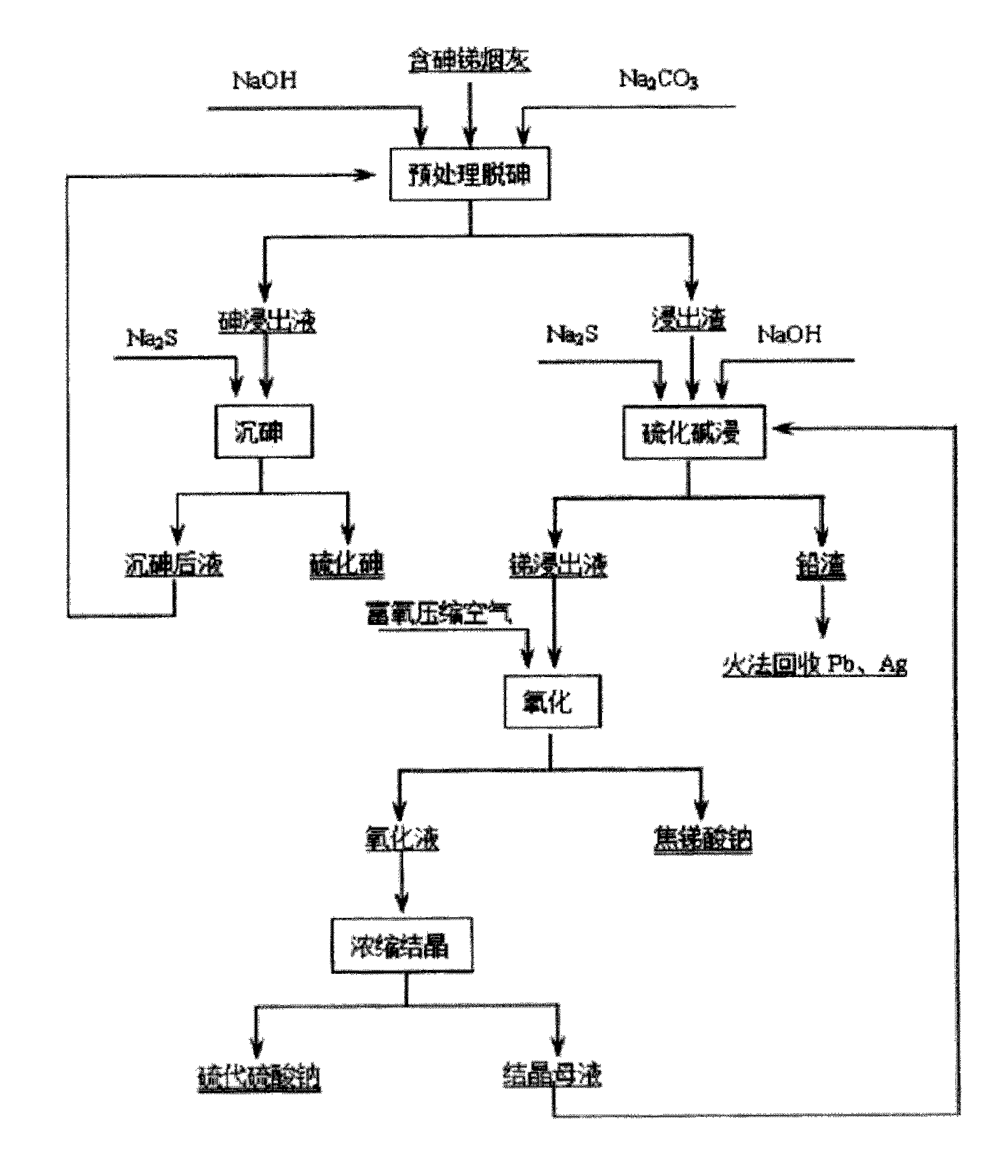
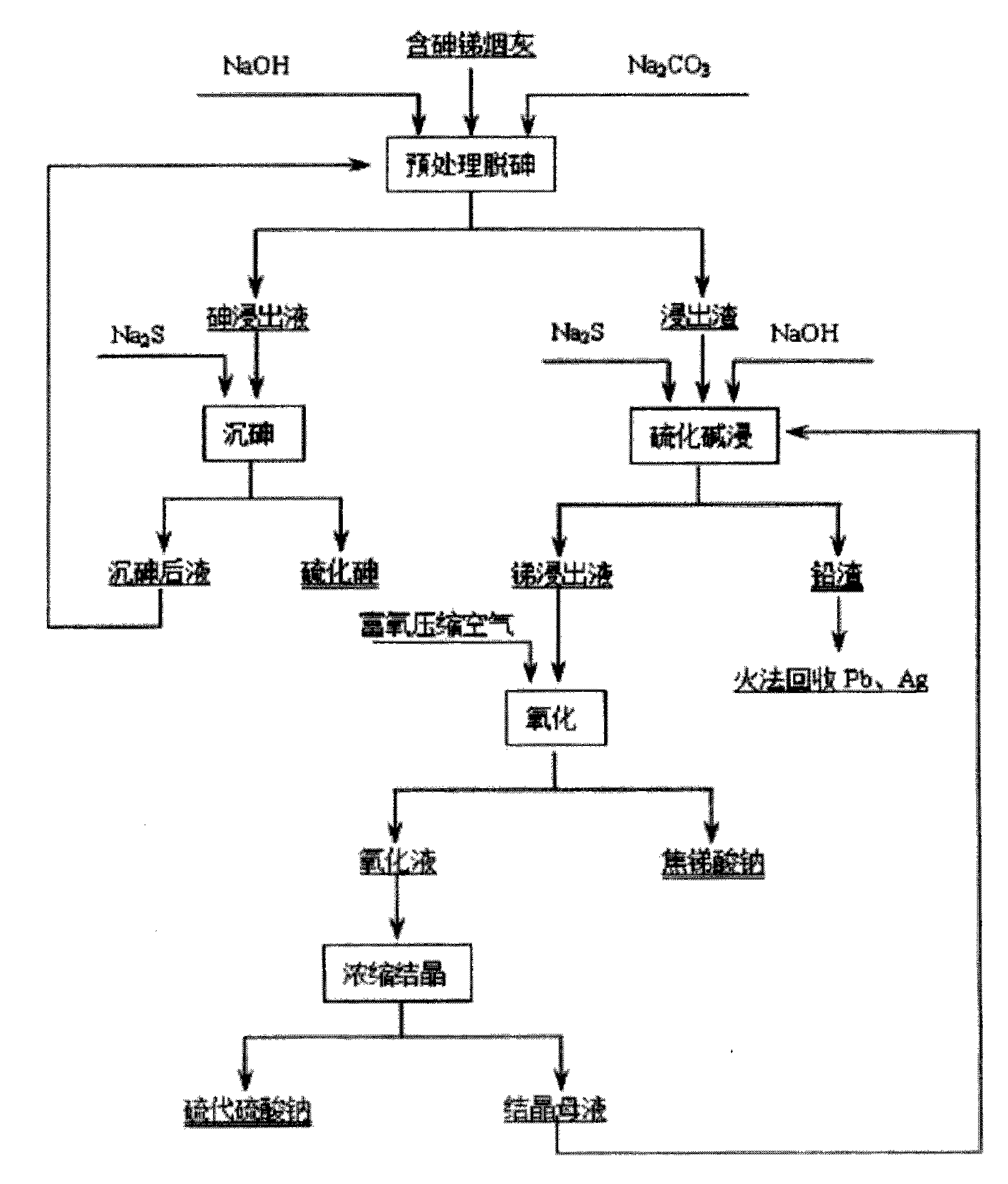
Process for preparing sodium pyroantimonate from arsenic- and stibium-containing smoke ash
A technology of sodium pyroantimonate and soot, which is applied in the field of hydrometallurgy to achieve the effects of low investment, simple equipment requirements, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take 200g of soot, containing As: 9.4%, Sb: 33.8%, Pb: 22.3%. During the pretreatment for arsenic removal, the solid-to-liquid ratio is 1:5, the NaOH concentration is 80g / L, and the NaOH concentration is 80g / L. 2 CO 3 The concentration is 20g / L, the temperature is 85-90°C, and the reaction time is 2 hours to control the reaction conditions. The treatment effects of As leaching rate of 95.42%, Pb leaching rate of 6.36%, and Sb leaching rate of 4.87% are achieved. When arsenic is precipitated by sodium sulfide in the leaching solution, the temperature is 70°C, the amount of sodium sulfide added is 1.5 times the mass of arsenic in the solution, the precipitation rate of arsenic is 89.73%, and the content of As in arsenic sulfide is 51.82%. When the leaching slag is pretreated by sulfide leaching, the solid-to-liquid ratio is 1:8, and Na 2 The concentration of S is 120g / L, the concentration of NaOH is 20g / L, the temperature is 95°C, and the reaction time is 3 hours. The...
Embodiment 2
[0034] 200g of soot in this example contains As: 6.08%, Sb: 35.46%, and Pb: 21.39%. When pretreatment to remove arsenic, according to solid-liquid ratio 1:4, NaOH concentration 100g / L, NaOH 2 CO 3 Concentration of 10g / L, temperature of 90-95°C, and reaction time of 4 hours control the reaction conditions. The treatment effects of As leaching rate of 98.35%, Pb leaching rate of 7.12%, and Sb leaching rate of 5.25% are achieved. When sodium sulfide in the leaching solution precipitates arsenic, the temperature is 60°C, the amount of sodium sulfide added is twice the mass of arsenic in the solution, the arsenic precipitation rate is 93.74%, and the arsenic sulfide contains As: 48.68%. When alkali sulfide leaching pre-treats the leached slag, the solid-to-liquid ratio is 1:10, the Na2S concentration is 110g / L, the NaOH concentration is 10g / L, the temperature is 90°C, and the reaction time is 4 hours. The antimony leaching rate is 96.18%, and the lead slag rate is 99.43%. When ...
Embodiment 3
[0036] Take 200g of soot, containing As: 9%, Sb: 35.2%, Pb: 21.2%. When pretreatment to remove arsenic, according to solid-liquid ratio 1:3; NaOH concentration 40g / L, NaOH 2 CO 3Concentration of 40g / L, temperature of 80-90°C and reaction time of 0.5 hours control the reaction conditions. The treatment effects of As leaching rate of 95.42%, Pb leaching rate of 6.36%, and Sb leaching rate of 4.87% are achieved. When arsenic is precipitated by sodium sulfide in the leaching solution, the temperature is 50°C, the amount of sodium sulfide added is twice the mass of arsenic in the solution, the precipitation rate of arsenic is 89.73%, and the content of As in arsenic sulfide is 51.82%. When the leaching slag is pretreated by sulfide leaching, the solid-to-liquid ratio is 1:4, and Na 2 The concentration of S is 80g / L, the concentration of NaOH is 50g / L, the temperature is 80°C, and the reaction time is 2 hours. The antimony leaching rate is 95.37%, and the lead slag rate is 99.65...
PUM
| Property | Measurement | Unit |
|---|---|---|
| stone rate | aaaaa | aaaaa |
| stone rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com