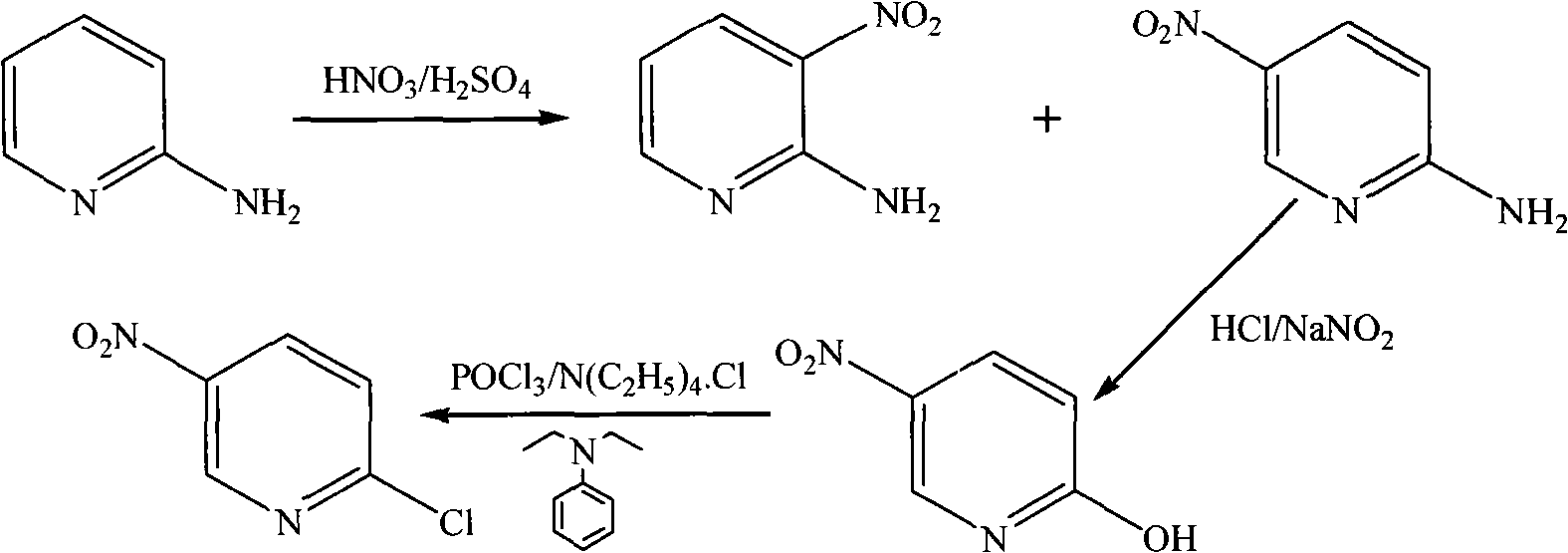
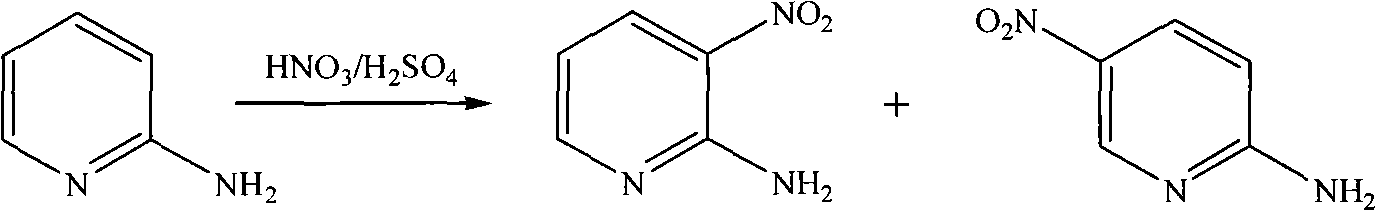
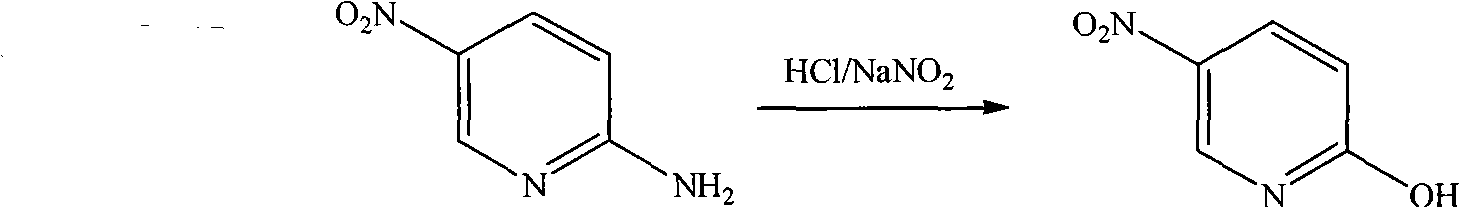
Method for preparing 2-chloro-5-nitropyridine
A technology of nitropyridine and aminopyridine, which is applied in the field of preparation of 2-chloro-5-nitropyridine, can solve the problems of high proportion of by-product 2-amino-3-nitro, low yield and the like, and achieves a total yield. High rate, less by-products, mild reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0017] Step 1, the synthesis of 2-amino-5 nitropyridine
[0018] Under stirring, add 2-aminopyridine (60.2 g, 0.639 mol) into concentrated sulfuric acid (150 mL) at a controlled temperature < 10° C. After the 2-aminopyridine is completely dissolved, keep the temperature < 30° C., and add concentrated sulfuric acid ( 95mL) and fuming nitric acid (95mL, 2.37mol), the dropwise addition was completed, and the temperature was kept at 25-30°C and stirred for 40min, then the temperature was raised to 55-65°C, and kept for 11h. The reaction of the raw material 2-aminopyridine was detected by TLC. After the end, pour the reaction solution into crushed ice (1000g), adjust the pH=5.5 to 6.0 with 50wt.% aqueous sodium hydroxide solution, filter the precipitate with suction, wash the filter cake with ice water (100mL×2), and dissolve the filter cake in 10wt.% hydrochloric acid aqueous solution (200mL), suction filtration, remove the surface oil, filtrate with 50wt.% sodium hydroxide aqueou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com