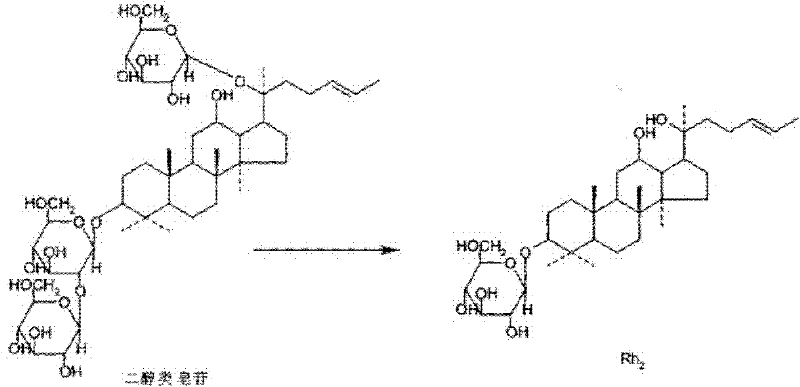
Cylindrocarpon didymium and method for preparing ginsenoside Rh2 by using same
A technology of ginsenoside and twin columns, which is applied in the field of preparing rare ginsenoside Rh2, can solve the problems of poor selectivity, difficult separation of mixtures, economic impracticality, etc., and achieve the effect of low cost and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Isolation and screening of bacterial strains
[0038] 1. Isolation of ginseng pathogenic bacteria: Rinse the diseased fresh ginseng with tap water, blot the water with filter paper, then soak it with 0.1% mercury liter for 1-1.5 minutes, rinse it with sterile water for 4-5 times, and then use Soak in 75% alcohol for 1 to 1.5 minutes, and then rinse with sterile water for 3 to 4 times. After the treatment, under aseptic conditions, the ginseng root tissue was cut into small pieces (4mm×4mm) with sterilized surgical scissors, and placed on PDA plates with a diameter of 9cm. After 5-7 days, observe whether there are colonies around the tissue block. After the colonies are produced, pick mycelia and inoculate them on the PDA slant medium for purification and preservation for future use.
[0039] Medium: potato dextrose agar medium (PDA medium): 200 g of potatoes, 18 g of glucose, 18 g of agar, 1000 mL.
[0040] The ginseng (Panax ginseng C.A.Mey) used in th...
Embodiment 2
[0050] Embodiment 2: conversion control experiment
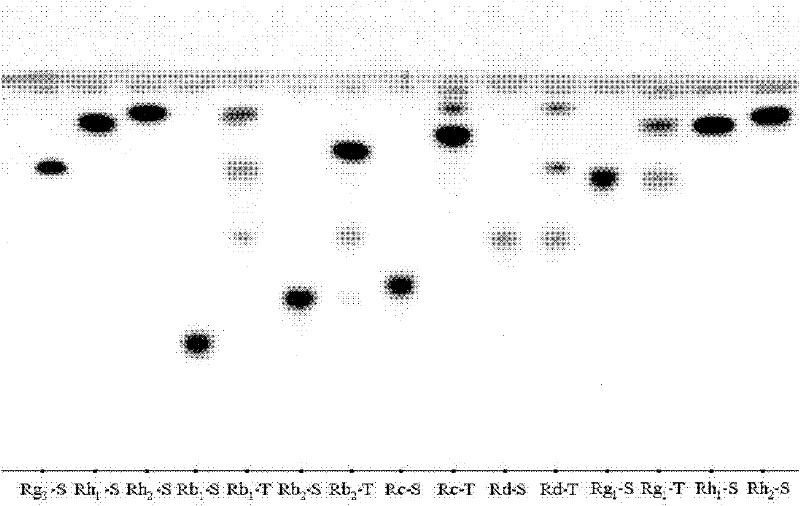
[0051] Adopt the method of embodiment 1 step 2 (2), the conversion selectivity of ginsenoside substrate and product of Cylindrospora bispora (CGMCCNo.4681) obtained by test screening, through TLC detection, the result is as attached image 3 Shown:
[0052] It can be seen that the protodiol saponin Rb 1 , Rb 2 . 1 , Rb 2 , Rc, and Rd all react, and for Rb 1 The conversion effect of is strong, and according to the Rf value, it can be preliminarily judged that the main product is Rh 2 .
Embodiment 3
[0053] Embodiment 3: solid transformation method transforms ginsenoside Rb 1 Preparation of Rh 2
[0054] ① Preparation of solid transformation medium (PDA): Each 100mL medium contains 20g of potato, 1.8g of glucose, 1.8g of agar, and ginsenoside Rb 1 ; Deionized water preparation, 121 ℃, 1.0kPa autoclave for 30min.
[0055] ② Spot the activated Cylindrospora bispora (CGMCC No.4681) to the above-mentioned 1 cultured on PDA medium at 25°C for 5-7 days.
[0056] ③Product extraction and separation: Soak the culture in n-butanol and fully extract; the extract is centrifuged at 10,000r / min for 1min, filtered through a microporous membrane with a diameter of 0.22μm, and the target product is separated by HPLC.
[0057] HPLC conditions: flow rate: 0.6mL / min; detection wavelength: 203nm; column temperature 35°C; mobile phase: A is acetonitrile, B is high-purity water;
[0058] Gradient elution mobile phase ratio:
[0059] 0min, A is 20%, B is 80%;
[0060] 18min, A is 40%, B is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com