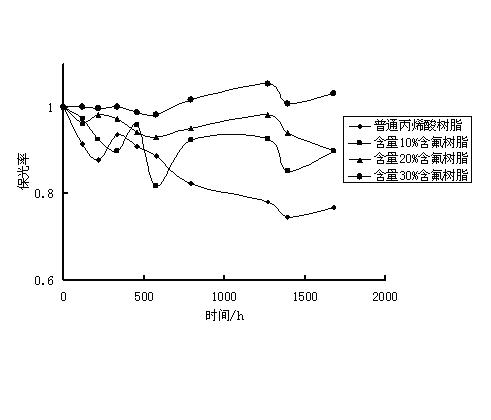
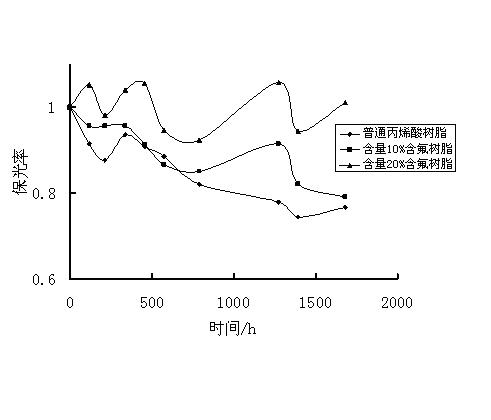
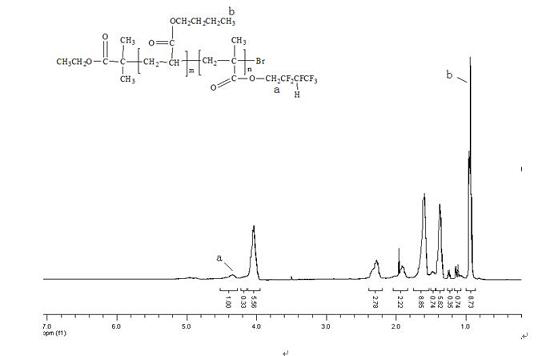
Fluorocoating resin and preparation method thereof
A technology of fluorine-containing coatings and resins, applied in the direction of polyester coatings, epoxy resin coatings, polyurea/polyurethane coatings, etc., can solve the problems of increased resin costs, achieve cost reduction, less fluorine-containing monomers, and compatibility good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: (Molecular weight 5000, block mass ratio of 1:1 butyl acrylate and hexafluorobutyl methacrylate block copolymer)
[0033] In a 100ml four-necked flask, add 1.164g (0.006mol) initiator α-bromoisobutyrate ethyl ester, 15g (0.117mol) butyl acrylate monomer, 0.168g (0.00117mol) catalyst CuBr, 0.405g (0.00234 mol) Ligand PMDETA, 5.25g solvent toluene, mix well. The system was evacuated and filled with nitrogen, and stirred and reacted in an oil bath at 70°C. After 8 hours, add 15 g (0.06 mol) hexafluorobutyl methacrylate, 0.0864 g (0.0006 mol) CuBr, 0.212 g (0.0012 mol) ligand PMDETA to the flask, pump again and fill with nitrogen, in an oil bath at 80 ° C The stirring reaction was continued for 12 hours, and the reaction was completed. After the catalyst and solvent were removed, a light yellow translucent viscous product was obtained, with a product quality of 26 g and a yield of 86.7%. The product was tested by GPC, and the test results are shown in Table 1...
Embodiment 2
[0034] Example 2: (Molecular weight 5000, block mass ratio of 4:1 butyl acrylate and hexafluorobutyl methacrylate block copolymer)
[0035]In a 100ml four-necked flask, add 0.97g (0.005mol) initiator α-bromoisobutyrate ethyl ester, 20g (0.156mol) monomer butyl acrylate, 0.225g (0.00156mol) catalyst CuBr, 0.552g (0.00312 mol) ligand PMDETA, 6g solvent toluene, mix well. The system was evacuated and filled with nitrogen, and stirred and reacted in an oil bath at 60°C. After 8 hours, add 5g (0.02mol) hexafluorobutyl methacrylate, 0.0288g (0.0002mol) CuBr, 0.0708g (0.0004mol) ligand PMDETA to the flask. The stirring reaction was continued for 10 hours, and the reaction was completed. After removing the catalyst and the solvent, a light yellow translucent viscous product was obtained, with a product quality of 22 g and a yield of 88%. The product was tested by GPC, and the test results are shown in Table 1. The butyl acrylate segment and the hexafluorobutyl methacrylate segmen...
Embodiment 3
[0036] Example 3: (Molecular weight 5000, block mass ratio of 9:1 butyl acrylate and hexafluorobutyl methacrylate block copolymer)
[0037] In a 100ml four-necked flask, add 0.776g (0.004mol) initiator α-bromoisobutyrate ethyl ester, 18g (0.141mol) monomer butyl acrylate, 0.203g (0.00141mol) catalyst CuBr, 0.499g (0.00282 mol) Ligand PMDETA, 5.4g solvent ethyl acetate, mix well. The system was evacuated and filled with nitrogen, and stirred and reacted in an oil bath at 60°C. After 8 hours, add 2 g (0.008 mol) hexafluorobutyl methacrylate, 0.0115 g (0.00008 mol) CuBr, 0.028 g (0.00016 mol) ligand PMDETA to the flask. The stirring reaction was continued for 8 hours, and the reaction was completed. After the catalyst and solvent were removed, a light yellow translucent viscous product was obtained, with a product quality of 17.4 g and a yield of 87%. The product was tested by GPC, and the test results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com