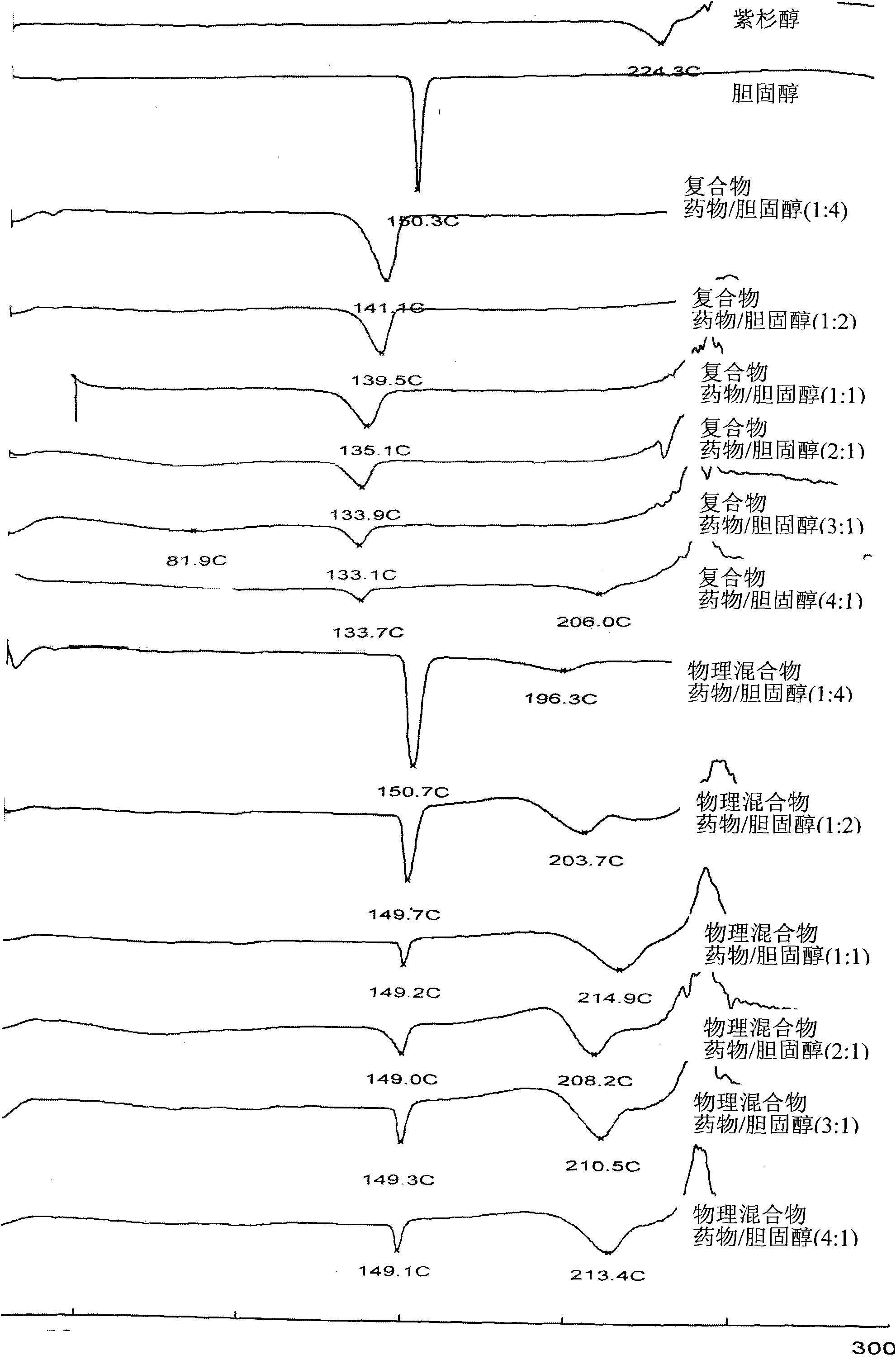
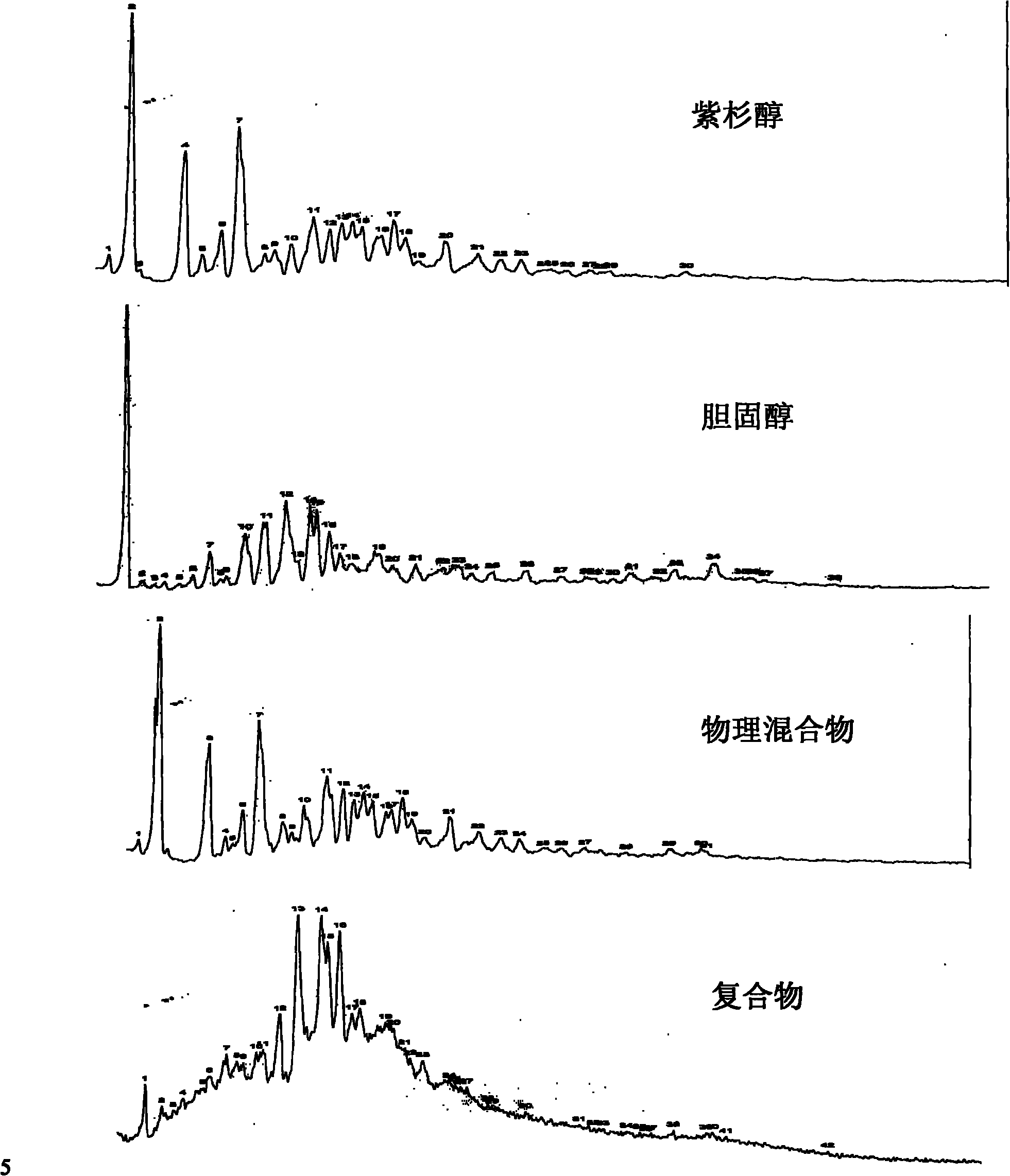
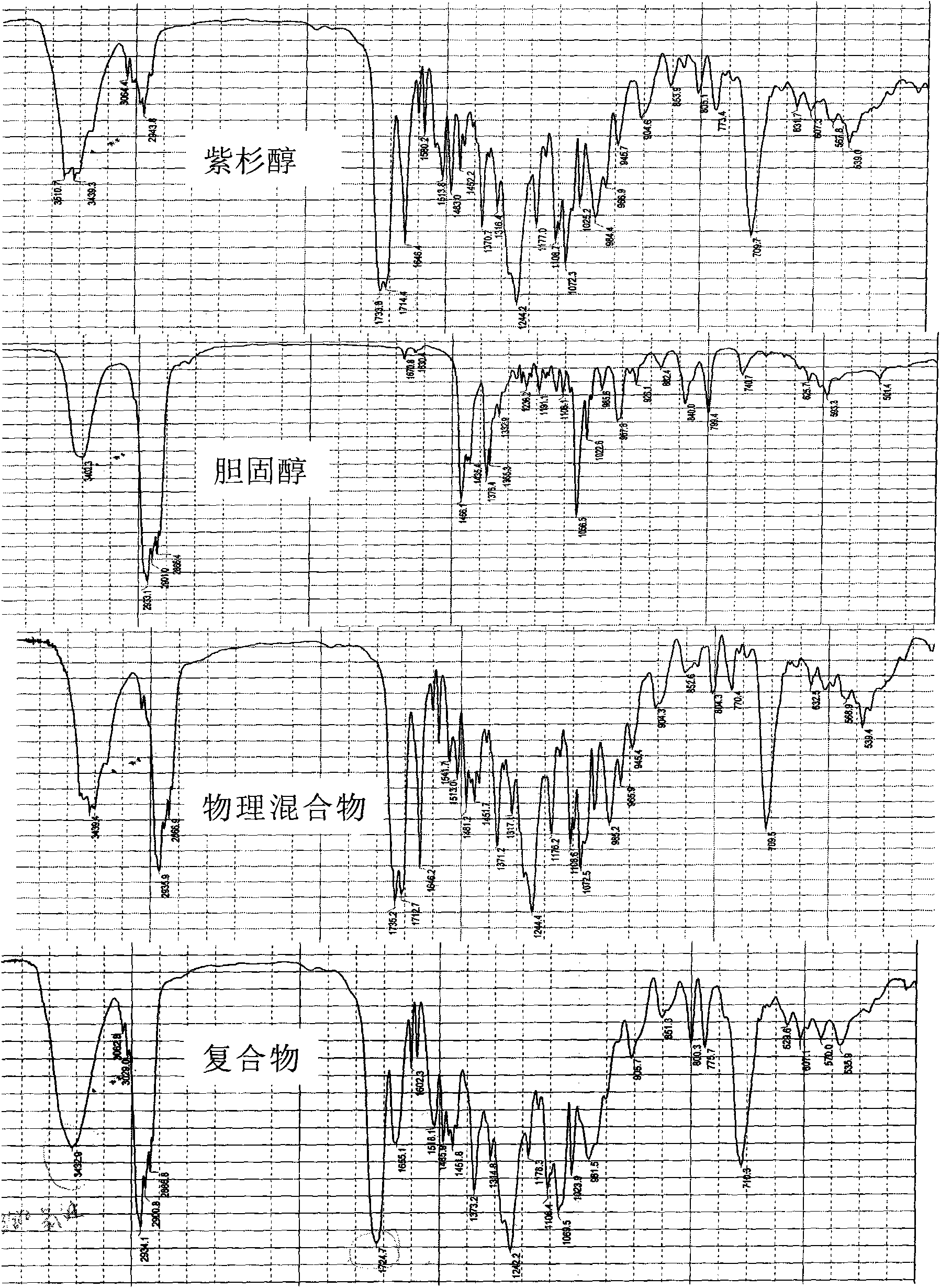
Taxol-cholesterin complex
A cholesterol and paclitaxel technology, which is applied in the directions of drug combinations, medical preparations of inactive ingredients, and pill delivery, etc., can solve the problems of preparation and storage of microemulsions, poor stability of submicroemulsions, and potential safety hazards, etc. Improve the encapsulation efficiency and stability, stable quality, and the effect of not easy to break demulsification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 paclitaxel cholesterol complex
[0040] Take 9.0g of paclitaxel and 0.81g of cholesterol in a rotary evaporator, add 3000ml of acetone to dissolve them, stir at 40°C for 1 hour, transfer to a rotary evaporator, remove the solvent by rotary evaporation, and decompress at 40°C After vacuum drying for 12 hours, 9.2 g of paclitaxel-cholesterol complex was obtained with a yield of 93.8%.
Embodiment 2
[0041] Embodiment 2 paclitaxel cholesterol complex
[0042] Take 9.0g of paclitaxel and 0.99g of cholesterol in a rotary evaporator, add 3000ml of tetrahydrofuran to dissolve them, stir at 25°C for 1 hour, transfer to a rotary evaporator, remove the solvent by rotary evaporation, and decompress at 25°C After vacuum drying for 12 hours, 9.5 g of paclitaxel-cholesterol complex was obtained with a yield of 95.0%.
Embodiment 3
[0043] Embodiment 3 paclitaxel cholesterol complex
[0044] Take 8.5g of paclitaxel and 1.275g of cholesterol in a conical flask, add 3000ml of tetrahydrofuran to dissolve them, stir at 45°C for 1.5 hours, transfer to a rotary evaporator, remove the solvent by rotary evaporation, and depressurize and vacuum at 45°C After drying for 16 hours, 9.3 g of paclitaxel-cholesterol complex was obtained with a yield of 95.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com