Method for preparing calycosin-7-O-beta-D-glucoside and ononin chemical reference products synchronously
A technique for chemistry of isoflavone glycosides and anthosides, applied in chemical instruments and methods, organic chemistry, preparation of sugar derivatives, etc., can solve the problems of cumbersome process, large loss of recrystallization, and research on preparation of isoflavone glycosides and formononetin There are no problems such as literature reports, and the effect of simple process and high yield is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Silica gel column chromatography enrichment:
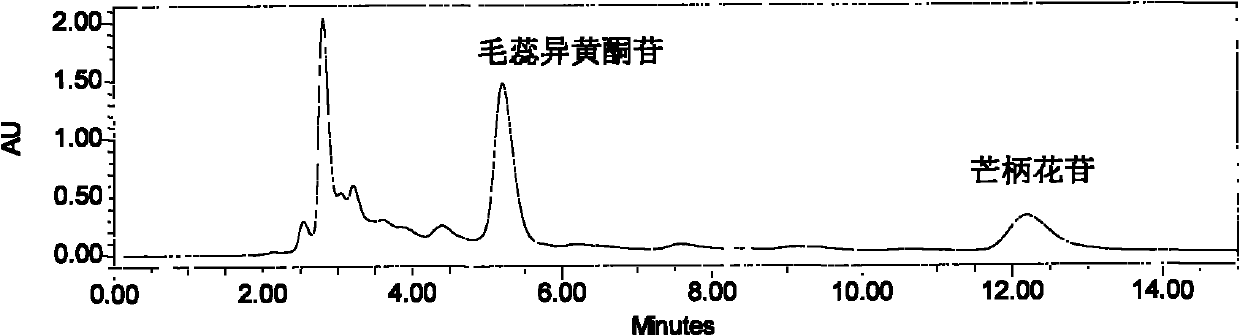
[0028] Astragalus ethanol extract 100g, separated by silica gel decompression chromatography (21cm*12cm), eluted with ethyl acetate:methanol (9:1) of 5 times the column volume, 500ml of each fraction was collected, and detected by thin layer chromatography, in which the developer Ethyl acetate: methanol: water (9: 1: 0.5), the color developer is a sulfuric acid ethanol solution with a volume concentration of 5%, and the fractions with an Rf value of 0.4-0.6 are combined and dried under reduced pressure to obtain 750 mg of a brown powder ( figure 1 );
[0029] 2) Resin column removal of impurities:
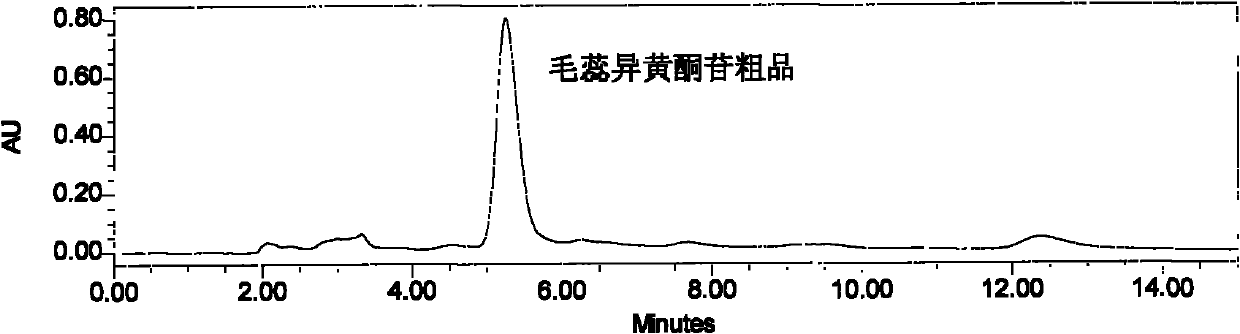
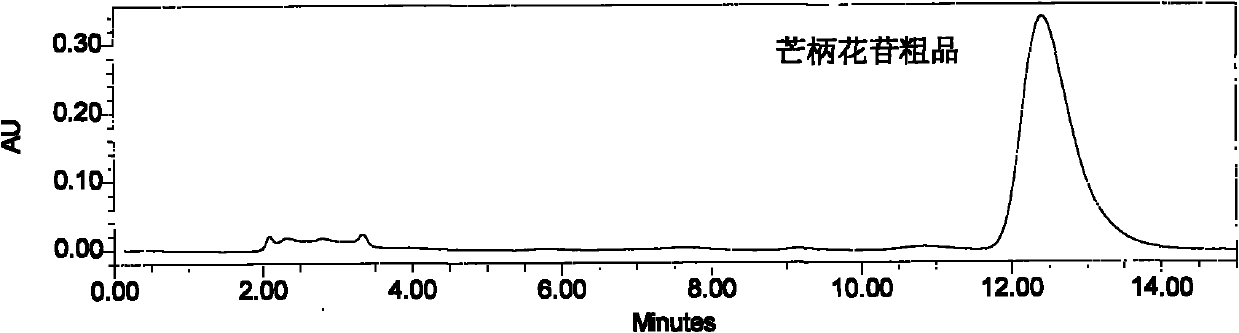
[0030] The tan powder is dissolved with 10% ethanol solution, filtered through four layers of gauze, and the supernatant liquid is separated on a HPD-100 resin column, wherein the sample: the resin ratio is 1: 6 (g: ml), respectively with 5 times the column volume of water, 25 % ethanol solution, 45% ethanol solution for el...
Embodiment 2
[0034] 1) Silica gel column chromatography enrichment:
[0035] Astragalus ethanol extract 1000g, separated by silica gel column chromatography (8cm*80cm), eluted with ethyl acetate:methanol (8:2) of 4 times the column volume, 500ml of each fraction is collected, and detected by thin-layer chromatography, wherein the developer is Ethyl acetate: methanol: water (9:1:0.5), the developer is 5% sulfuric acid ethanol solution, the fractions with Rf value of 0.4-0.6 are combined, and dried under reduced pressure to obtain 7.4 g of brown powder;
[0036] 2) Resin column removal of impurities:
[0037] The brownish yellow powder was dissolved with 25% ethanol solution, filtered through four layers of gauze, and the clear liquid was separated on a Diaion resin column, wherein the sample: resin ratio was 1:5 (g:ml), and the water and 30% ethanol were used for 4 times the column volume respectively. solution and 50% ethanol solution, respectively collected 30% ethanol eluate, concentrat...
Embodiment 3
[0041] 1) Silica gel column chromatography enrichment:
[0042] Astragalus ethanol extract 2000g, separated by silica gel column chromatography (9.5cm*120cm), eluted with 3 times column volume of ethyl acetate:methanol (7:3), 500ml of each fraction was collected, and detected by thin layer chromatography, in which the developer Ethyl acetate: methanol: water (9:1:0.5), the developer is 5% sulfuric acid ethanol solution, the fractions with Rf value of 0.4-0.6 are combined, dried under reduced pressure to obtain 15 g of brown powder;
[0043] 2) Resin column removal of impurities:
[0044] The tan powder was dissolved with 10% ethanol solution, filtered through four layers of gauze, and the supernatant liquid was separated on a HPD-100 resin column, wherein the sample: the resin ratio was 1: 6 (g: ml), and 3 times the column volume of water, 35 % ethanol solution and 55% ethanol solution for elution, respectively collected 35% ethanol eluate, concentrated and dried to obtain 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com