Method for preparing N-hydroxymethyl-3-(dimethoxyphosphoryl) propionamide
A technology of dimethoxyphosphoryl and hydroxymethyl, which is applied in the field of flame retardants, can solve the problems of low reaction efficiency, high preparation cost, and long reaction time, and achieve high reaction efficiency, short reaction time, and low reaction temperature Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention discloses a preparation method of N-hydroxymethyl-3-(dimethoxyphosphoryl)propionamide, comprising:
[0028] Acrylamide, dimethyl phosphite, and a catalyst are reacted in a solvent to obtain dimethyl (3-amino-3-acylpropyl) phosphonate, and the catalyst is a non-acidic compound of an alkaline earth metal;
[0029] Reaction of the dimethyl(3-amino-3-acylpropyl)phosphonate with formaldehyde affords N-methylol-3-(dimethoxyphosphoryl)propanamide.
[0030] The acrylamide, dimethyl phosphite and catalyst react in a solvent to obtain dimethyl (3-amino-3-acylpropyl) phosphonate, and the reaction formula is as shown in formula I, and the dimethyl phosphite The molar ratio of fat and acrylamide is preferably 1~1.2:1, more preferably 1~1.1:1,
[0031]
[0032] The catalyst is a non-acidic compound of alkaline earth metal, preferably one or more of magnesium oxide, magnesium hydroxide, calcium oxide, calcium hydroxide, calcium hydride, strontium oxide, strontium hyd...
Embodiment 1
[0045] Add 5.517g of dimethyl phosphite (48.15mmol), 3.200g of acrylamide (45mmol), 0.180g of powdered magnesium oxide (4.5mmol), 10mL of methanol, 2mg of copper acetate, and a magnetic stirring bar into a 50mL three-necked flask. , a glass thermometer is installed in the three-necked bottle, and the bottle mouth is connected with a spherical condenser;
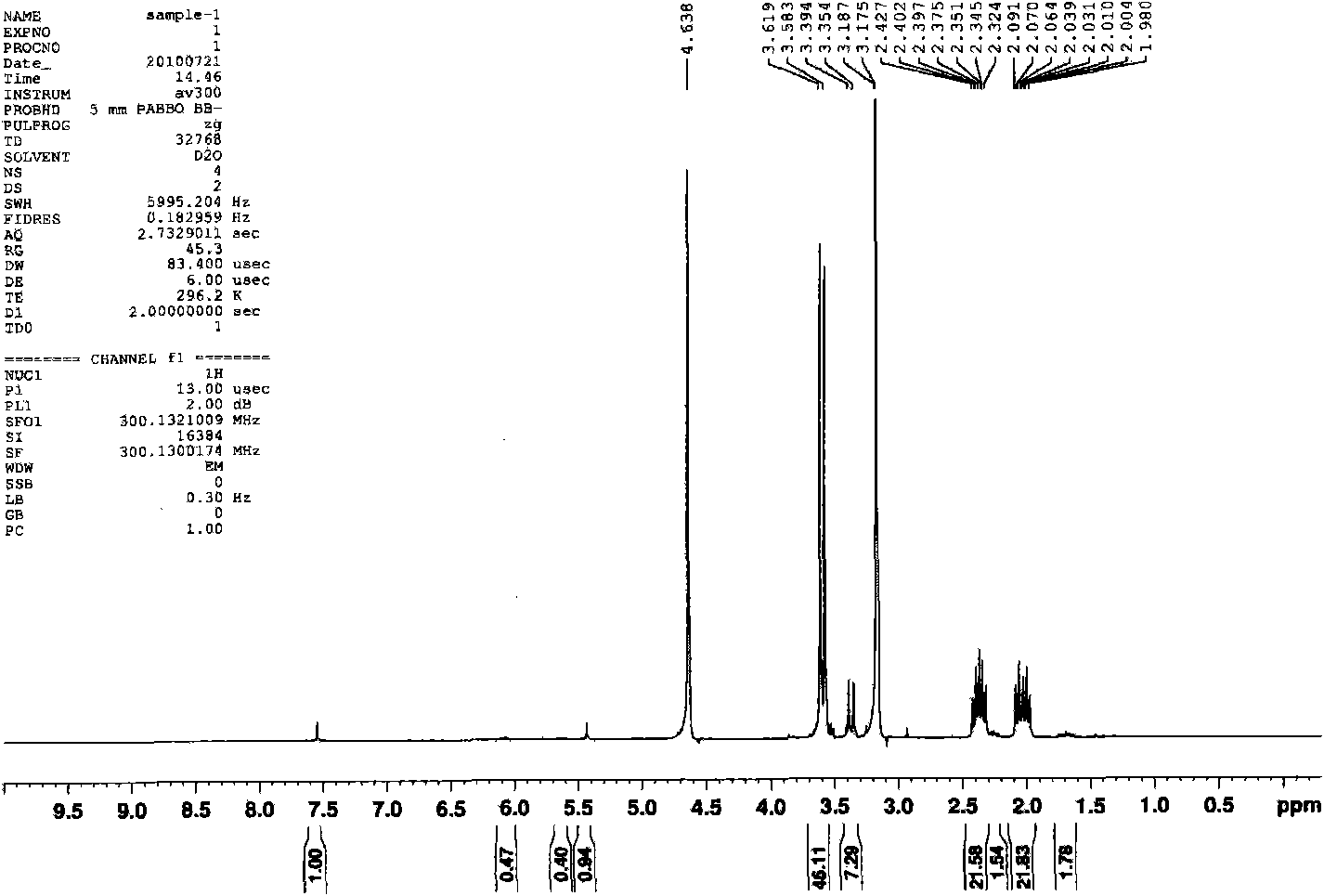
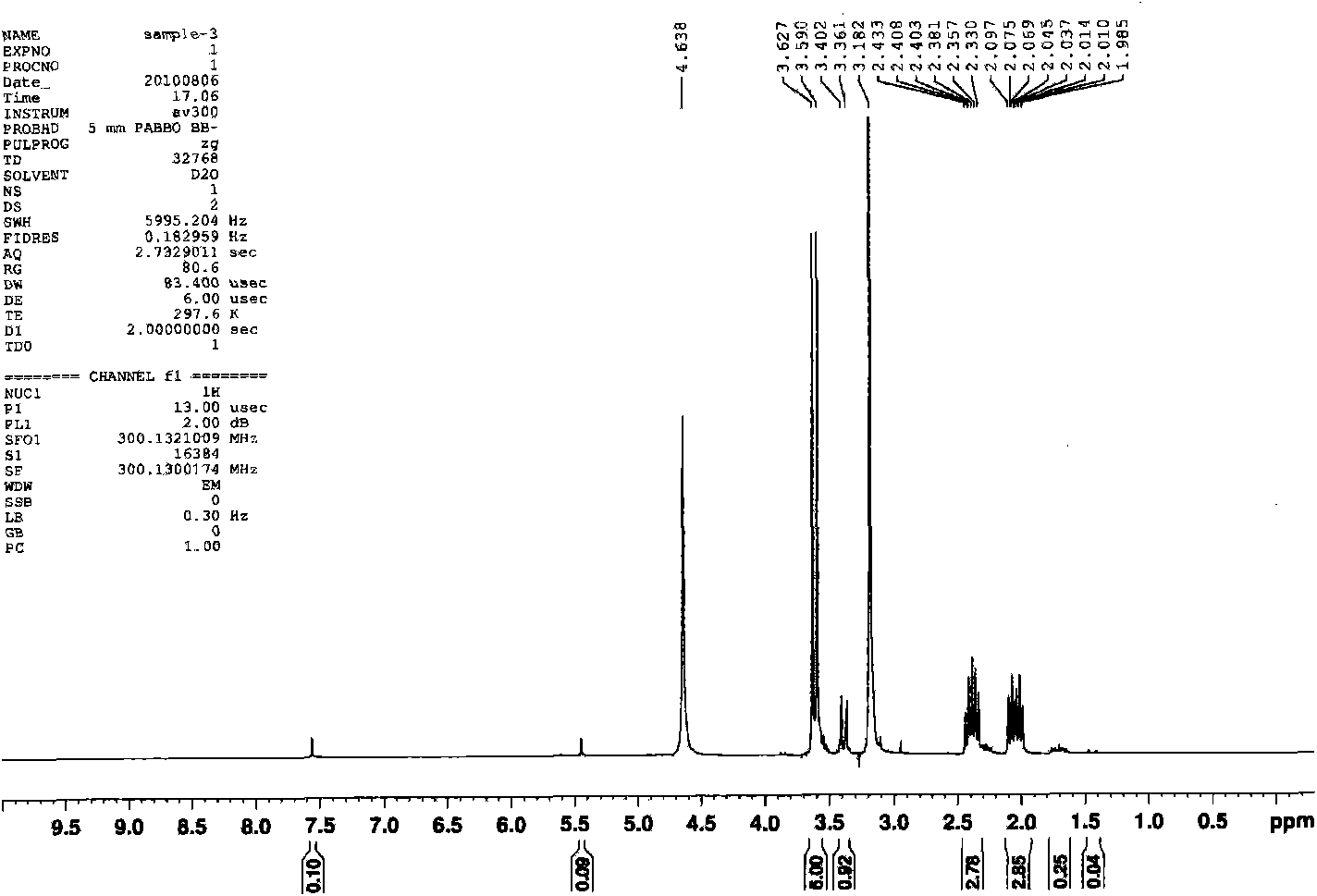
[0046] Pump out the air in the three-necked bottle once, fill it with ordinary nitrogen, then place the three-necked bottle in a preheated oil bath at 50 degrees Celsius, and magnetically stir the reaction system. The temperature of the system begins to rise in about 3 minutes, and the system boils when the internal temperature reaches 71 degrees Celsius. , then the temperature of the system began to decrease. After 20 minutes, the internal temperature of the system was 61 degrees Celsius. The reaction was stopped, and the reaction product was obtained. The reaction product was filtered, and the nuclear magnetic test was carri...
Embodiment 2
[0049] Add 22.294g of dimethyl phosphite (240.75mmol), 15.975g of acrylamide (225mmol), 0.900g of powdered magnesium oxide (22.5mmol), 50mL of methanol, 10mg of copper acetate, and a magnetic stirrer into a 250mL three-necked flask. , a glass thermometer is installed in the three-necked bottle, and the bottle mouth is connected with a spherical condenser;
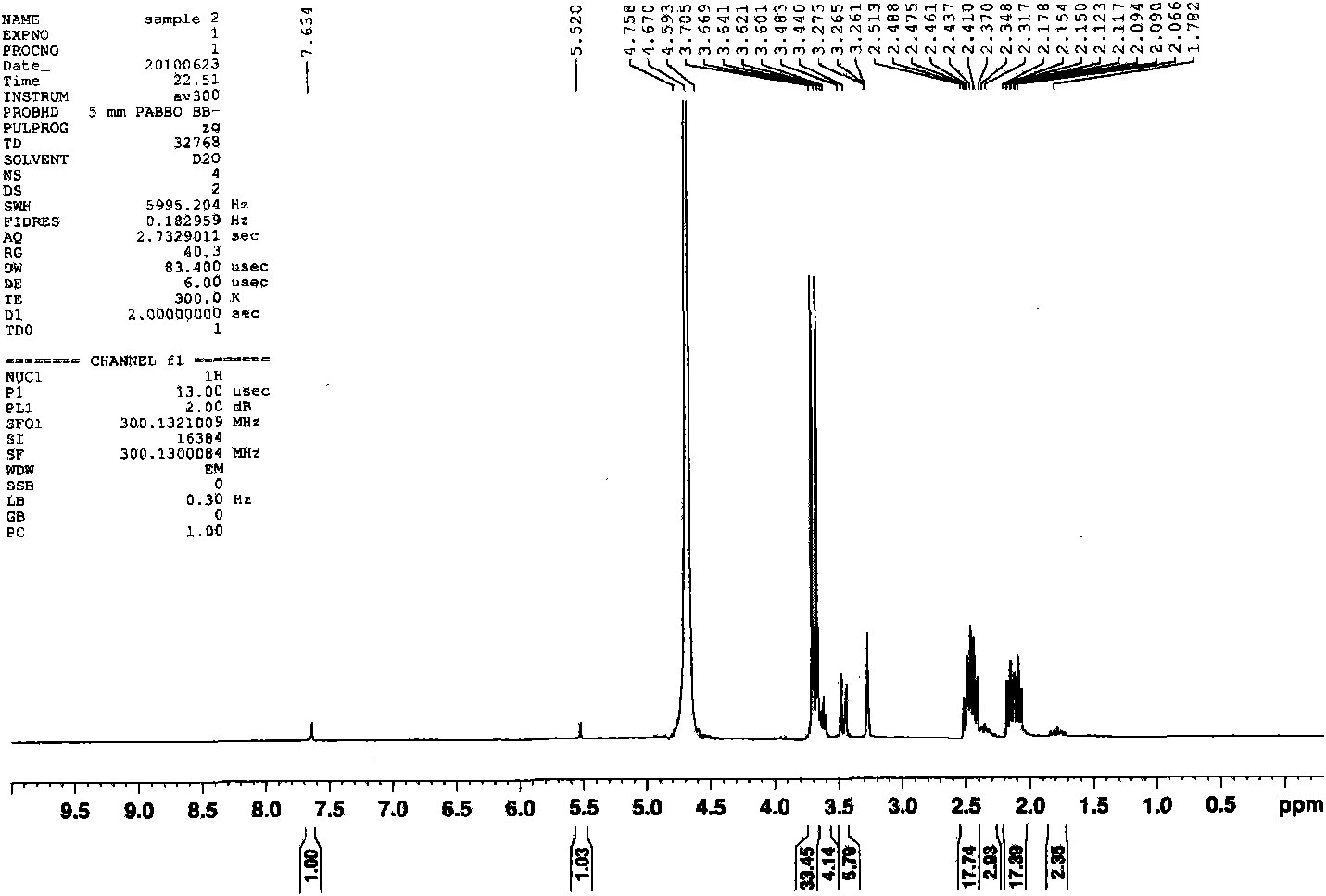
[0050] Pump out the air in the three-necked bottle once, fill it with ordinary nitrogen, then place the three-necked bottle in a preheated oil bath at 50 degrees Celsius, and magnetically stir the reaction system. The temperature of the system begins to rise in about 3 minutes, and the system boils when the internal temperature reaches 71 degrees Celsius. , then the temperature of the system began to decrease. After 20 minutes, the internal temperature of the system was 61 degrees Celsius. The reaction was stopped, and the reaction product was obtained. The reaction product was filtered, and the nuclear magnetic test was car...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com