Novel synthetic antithrombotic polypeptide, and preparation method and application thereof
An antithrombotic activity and antithrombotic drug technology, applied in the field of biomedicine, can solve problems such as small side effects, and achieve the effect of strong antithrombotic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 Preparation and separation and purification of novel antithrombotic polypeptide BNC
[0026] BNCs were prepared according to the following sequence.
[0027] The amino acid sequence of BNC is: pyroglutamic acid-asparagine-cysteine.
[0028] In this example, the solid-phase peptide synthesis technique was used for manual synthesis, and the synthesized peptide was cleaved with high-concentration TFA to obtain a crude product. After purification by HPLC reverse phase column, it was confirmed by mass spectrometry analysis. The specific experimental steps are as follows:
[0029] 1) Preparation of BNC (taking the preparation of 1.323 mmol of BNC as an example to illustrate the method of solid-phase synthesis of polypeptides)
[0030] The following resins, Fmoc-protected amino acids, condensation reagents, and cleavage reagents for preparing antithrombotic polypeptide BNC were all purchased from Shanghai Gil Biochemical Co., Ltd.
[0031] The preparation is c...
Embodiment 2B
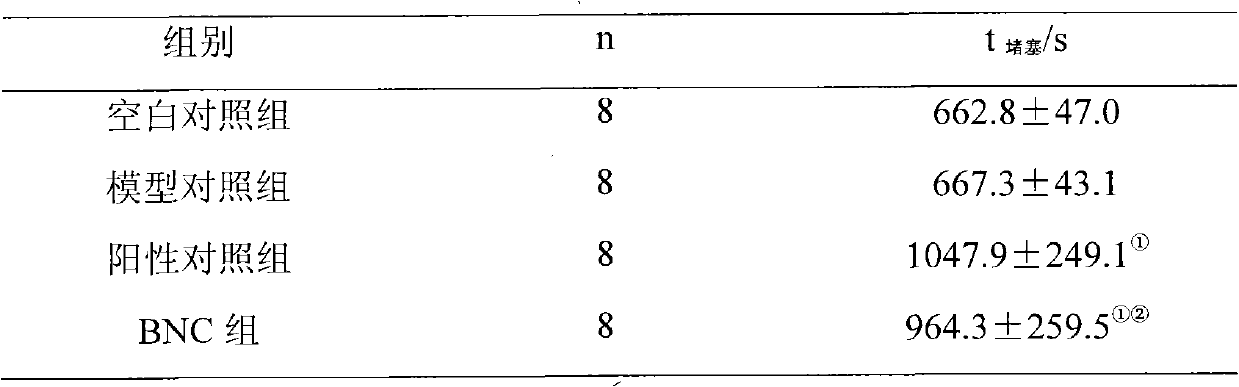
[0035] Example 2 Effect of BNC on Thrombosis after Electric Stimulation of Rat Common Carotid Artery Intima
[0036] 1) Preparation of sample solution
[0037] Before the positive control group was used, clopidogrel was diluted with normal saline to the required concentration of the drug solution (2mg / ml); the BNC group was dissolved with 0.2% L-arginine before use, and diluted with normal saline to the required concentration. Concentration of liquid medicine (2mg / ml). All groups were given tail vein administration with a volume of 10ml / kg.
[0038] 2) Preparation of animal models
[0039]Rats were intraperitoneally injected with 10 mg / L pentobarbital sodium solution (30 mg / kg), and fixed in a supine position after anesthesia. A midline incision was made in the neck, and the right common carotid artery was freed. The stimulating electrode of the BT87-3 experimental in vivo thrombosis measuring instrument was placed at the proximal end, and the temperature probe was placed a...
Embodiment 3B
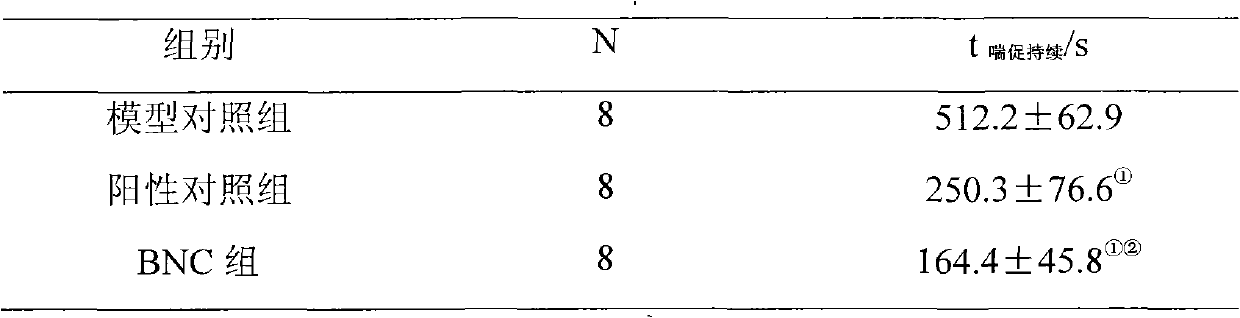
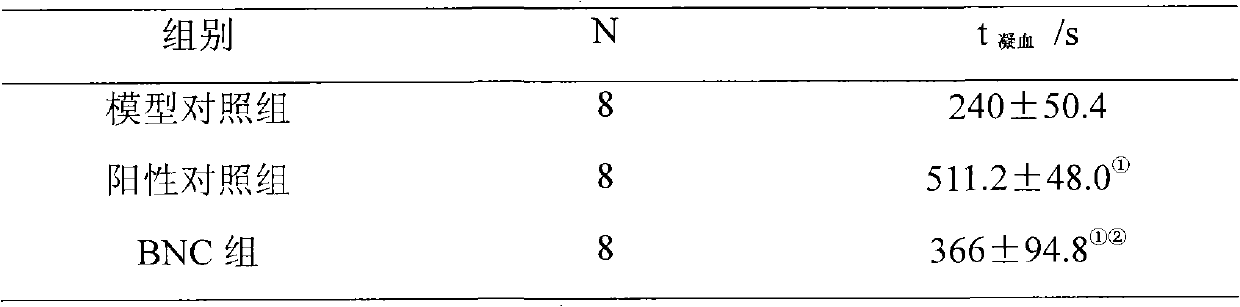
[0048] The effect of embodiment 3BNC on the mouse acute pulmonary thrombosis induced by ADP
[0049] 1) Preparation of sample solution
[0050] Before use, the positive control group diluted clopidogrel with normal saline into a drug solution of the required concentration (the dose was 4 mg / kg); before use, the BNC group was diluted with normal saline into the drug solution of the required concentration (the dose was 4 mg / kg). kg); the inducer ADP solution was diluted with pure water to the required concentration (200mg / kg) before use. All groups were given tail vein administration with a volume of 10ml / kg.
[0051] 2) Experimental steps
[0052] Take 24 healthy ICR mice, weighing 20-25g, half male and half male, and randomly divide them into 3 groups according to body weight, 8 mice in each group, that is, model control group, positive control group and BNC group. Each group was given tail vein administration with a volume of 10ml / kg, the model control group was given equa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com