Human-mouse chimeric monoclonal antibody for human von willebrand factor A3 region as well as preparation method and application of human-mouse chimeric monoclonal antibody
A hemophilia factor and monoclonal antibody technology, applied in the biological field, can solve the problems of increasing the probability of immune response, weakening of immune response, and patient's allergic reaction, so as to reduce cardiac ischemic events, prevent the formation of acute thrombosis, The effect of prolonged bleeding time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Construction of human-mouse chimeric monoclonal antibody eukaryotic expression plasmid.
[0044] 1. Construction of the heavy chain and light chain variable region cDNA of the monoclonal antibody SZ-123:
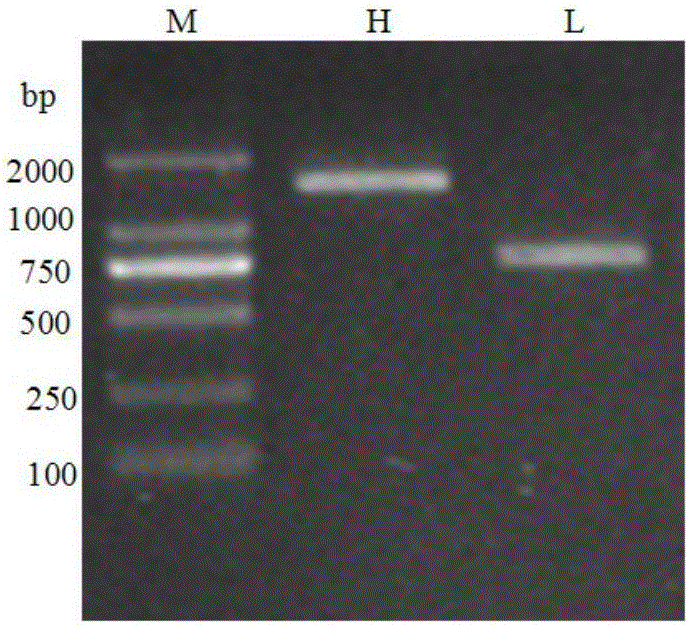
[0045] Using 5'-RACE technology, the variable region sequences of the heavy chain and light chain of the functional antibody were cloned from the hybridoma cell line SZ-123 secreting the mouse monoclonal antibody against the human vWFA3 region, in which the mouse monoclonal antibody SZ- The mRNA of 123 hybridoma cell lines was used as a template, and a set of heavy chain and light chain reverse transcription specific primers HGSP1 and KGSP1 were designed, the nucleotide sequences of which were shown in SEQ ID NO: 3 and SEQ ID NO: 4, respectively. The electrophoresis of the 5'-RACE product is as follows figure 1 As shown, the right side is the light chain (L) result, and the left side is the heavy chain (H) result.
[0046]
[0047] According to the cor...
Embodiment 2
[0052] Example 2: Expression, screening, suspension acclimatization and shake flask expression of chimeric monoclonal antibody.
[0053] 1. CHO-S cell electrotransfection method:
[0054] 20 μg of recombinant plasmids (pMH3-MHC-SZ123VH, pMH3-MHC-SZ123VK) were co-transformed into 3×10 6 CHO-S cells, electroporation reaction system: 200 μL, CHO-S cell suspension + 20 μg plasmid + 10 μg salmon sperm DNA, electroporation reaction conditions: 500V, 500us, 4 times.
[0055] 2. Screening of high expression cell lines:
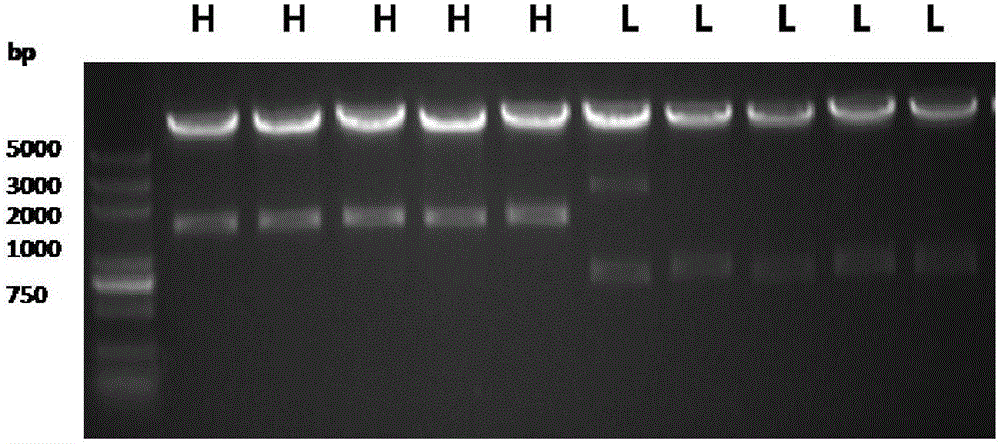
[0056] Spread the electroporated cell suspension in a Petri dish containing 10 mL of medium (DMEM / F12=1:1, containing 10% FBS). 2.4mg / LG418 (Geneticin) was used to screen the cells under pressure, and single clones were selected and transferred to a 96-well plate. When the clone grows to cover 80% of the bottom of the well, remove the FBS medium, add D / F medium (expression medium) at a dose of 100 μL / well, and detect the expression after 48 hours. Samples were dil...
Embodiment 3
[0067] Example 3: Purification of chimeric monoclonal antibody.
[0068] The chimeric monoclonal antibody was purified by protein A affinity chromatography, and the specific steps were as follows:
[0069] (1) Wash the protein A affinity chromatography column with 3 to 5 times the column volume of water;
[0070] (2) Equilibrate the protein A affinity chromatography column with 20mM phosphate buffer (PBS) (pH=7.0);
[0071] (3) Filter the cell culture supernatant containing the desired purified monoclonal antibody with a 0.45 μm filter membrane, and pump it into the protein A affinity column that has been equilibrated with PBS;
[0072] (4) Wash the column with 20mMPBS (pH=7.0) until OD 280 <0.01;
[0073] (5 ) Elute the target protein with 0.1M glycine-HCl buffer (pH=3.0), collect the elution peak, and adjust the collected antibody solution with 1M Tris-HCl buffer (pH=9.0) until the pH value is neutral;
[0074] (6) Dialyze and desalt with 10mMPBS (pH=7.2) to obtain pure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com