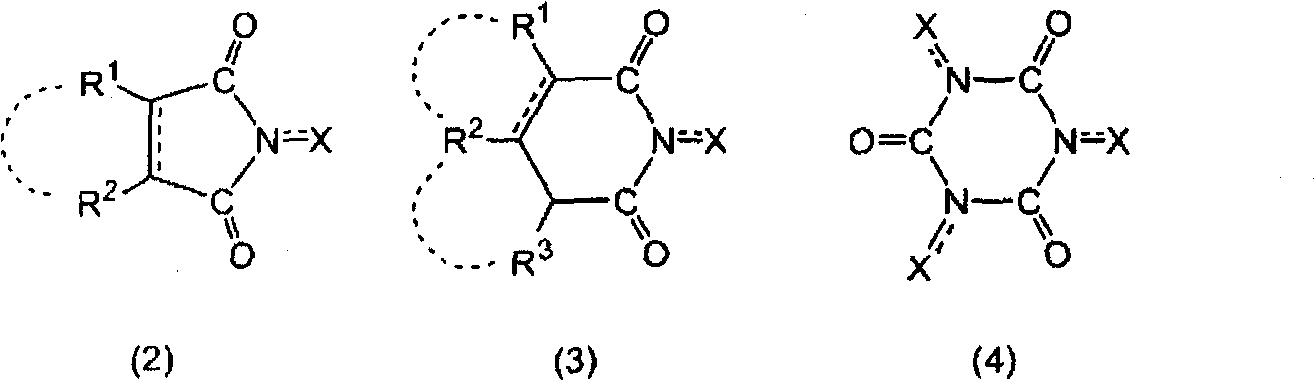
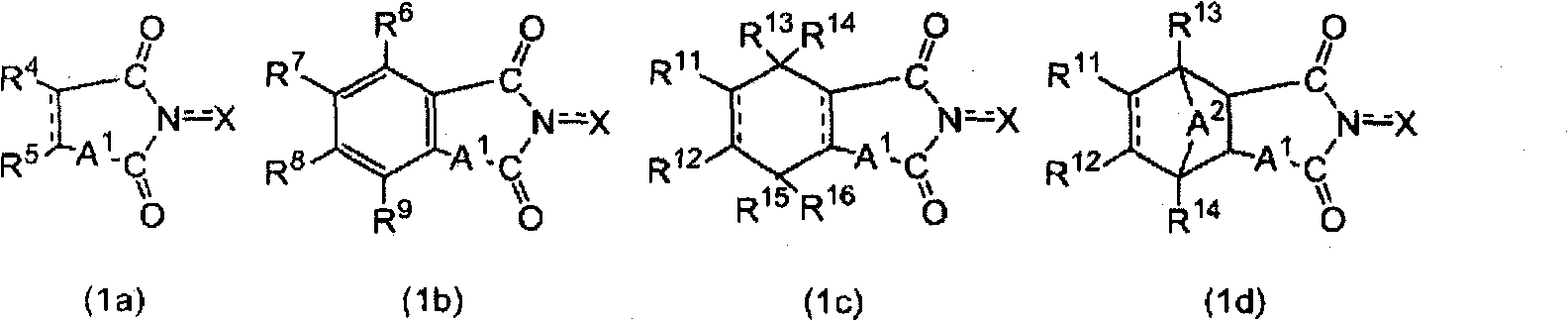
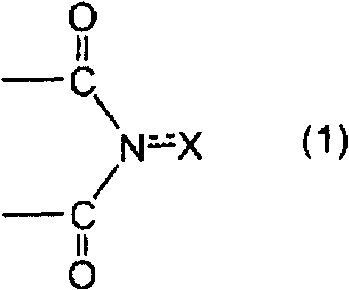Aromatic polycarboxylic acid manufacturing method
A technology of polycarboxylic acid and manufacturing method, applied in the direction of carboxylate preparation, organic chemical method, chemical instrument and method, etc., can solve the problems such as difficult to obtain high yield and obtain target compound, so as to improve selectivity and reduce residue Quantitative and efficient manufacturing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Embodiment 1 (reaction temperature 80 ℃ → 120 ℃ → 130 ℃)
[0123] Add 40 g (0.30 mol) of durene, 320 g of acetic acid, 0.19 g (0.7 mmol) of cobalt acetate (2 valence), 0.55 g (2.2 mmol) of manganese acetate (2 valence), zirconium sulfate into the air circulation reactor. 1.06g (3.0 mmol), the pressure was raised to 0.8MPa with nitrogen, and heated to 80°C.
[0124] Add 14.8 g (60 mmol) of N, N'-dihydroxypyromellitic acid diimide to 300 g of acetic acid to obtain a slurry, and start supplying the slurry and the gas obtained by mixing air and nitrogen into the reactor to trigger a reaction. The above-mentioned slurry was fed into the reactor by using a slurry pump for 5 hours, and the gas supply was adjusted so that the oxygen concentration in the outlet gas was 2-8%. After the reaction started, the reaction temperature was raised to 120° C. over 0.5 hour, and the above state was maintained for 1.5 hours. Here, samples were taken for HPLC analysis, and then the reactio...
reference example 1
[0127] Reference example 1 (reaction temperature 130°C → 160°C)
[0128] Add 40 g (0.30 mol) of durene, 320 g of acetic acid, 0.19 g (0.7 mmol) of cobalt acetate (2 valence), 0.55 g (2.2 mmol) of manganese acetate (2 valence), zirconium sulfate into the air circulation reactor. 1.06g (3.0mmol), the pressure was raised to 0.8MPa with nitrogen, and heated to 130°C.
[0129] Add 14.8 g (60 mmol) of N, N'-dihydroxypyromellitic acid diimide to 300 g of acetic acid to obtain a slurry, and start supplying the slurry and the gas obtained by mixing air and nitrogen into the reactor to trigger a reaction. The above-mentioned slurry was fed into the reactor using a slurry pump for 5 hours, and the gas supply was adjusted so that the oxygen concentration in the off-gas was 2 to 8%. After the reaction started, the reaction temperature was raised to 160° C. over 0.5 hour, and the above state was maintained for 4.5 hours. During the reaction, the gas and catalyst supply are adjusted as ne...
Embodiment 2
[0132] Example 2 (reaction temperature 80°C → 120°C → 130°C, pressure 2MPa)
[0133] Add 40 g (0.30 mol) of durene, 320 g of acetic acid, 0.19 g (0.7 mmol) of cobalt acetate (2 valence), 0.55 g (2.2 mmol) of manganese acetate (2 valence), zirconium sulfate into the air circulation reactor. 1.06g (3.0 mmol), the pressure was raised to 2MPa with nitrogen, and heated to 80°C.
[0134]Add 14.8 g (60 mmol) of N, N'-dihydroxypyromellitic acid diimide to 300 g of acetic acid to obtain a slurry, and start supplying the slurry and the gas obtained by mixing air and nitrogen into the reactor to trigger a reaction. The above-mentioned slurry was fed into the reactor by using a slurry pump for 5 hours, and the gas supply was adjusted so that the oxygen concentration in the outlet gas was 2-8%. After the reaction started, the reaction temperature was raised to 120° C. over 0.5 hour, and the above state was maintained for 1.5 hours. Here, samples were taken for HPLC analysis, and then th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com