Quality control method for Chinese medicinal preparation heat clearing and blood cooling pills
A quality control method, heat-clearing and blood-clearing technology, applied in the direction of medical formula, non-central analgesics, antipyretics, etc., can solve problems such as lack and identification means, increase detection effect, promote product sales, The effect of improving controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
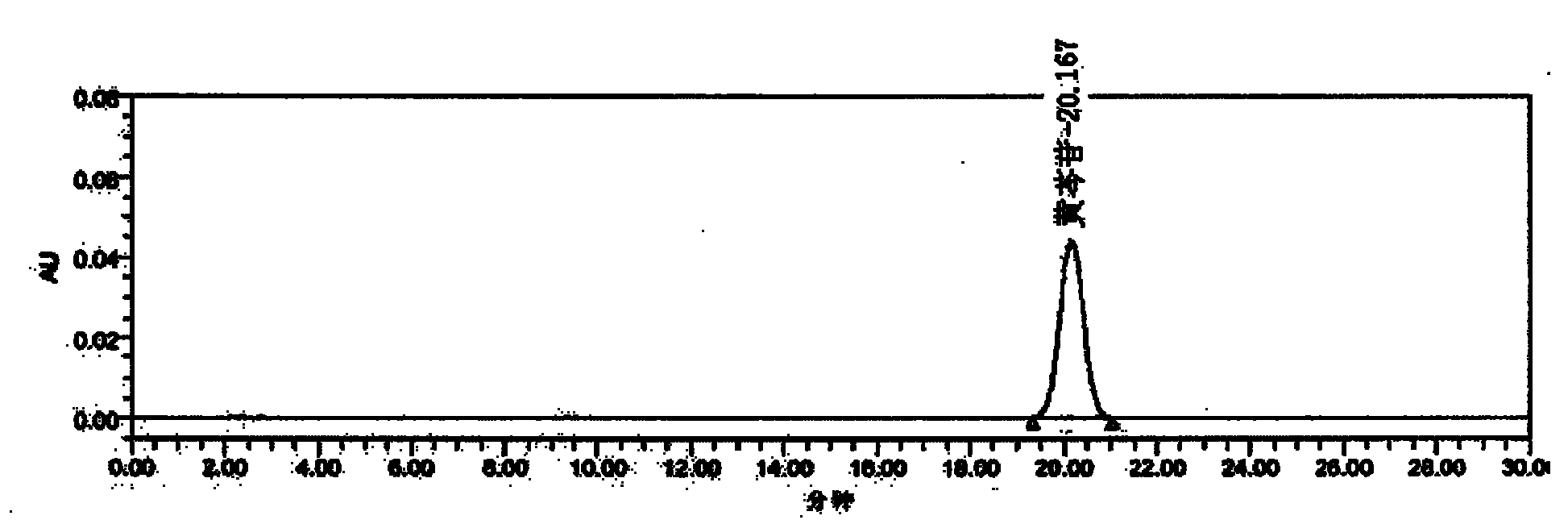
[0040] Preparation of Reference Substance Solution Take an appropriate amount of baicalin reference substance, accurately weigh it, add methanol to make a solution containing 0.1mg per 1ml, and obtain it.
[0041]Preparation of the test solution: Take 2g of the sample, grind it finely, get 0.2g of the powder, accurately weigh it, place it in a stoppered Erlenmeyer flask, accurately measure 100ml of 70% ethanol, weigh it, heat and reflux for 30 minutes, let it cool, Weigh the weight, make up the lost weight with 70% ethanol, shake well, filter, and take the filtrate to obtain the final product.
[0042] Determination method Precisely draw 5 μl each of the reference substance solution and the test solution, inject it into the liquid chromatograph, measure it, and obtain it.
[0043] This product contains not less than 24mg of baicalin per gram.
[0044] Functions and indications: nourishing yin, clearing heat, cooling blood. It is used for pregnant women with excessive upper b...
Embodiment 2
[0047] Embodiment 2 Qingreliangxue Wan Quality Standard Drafting Instructions:
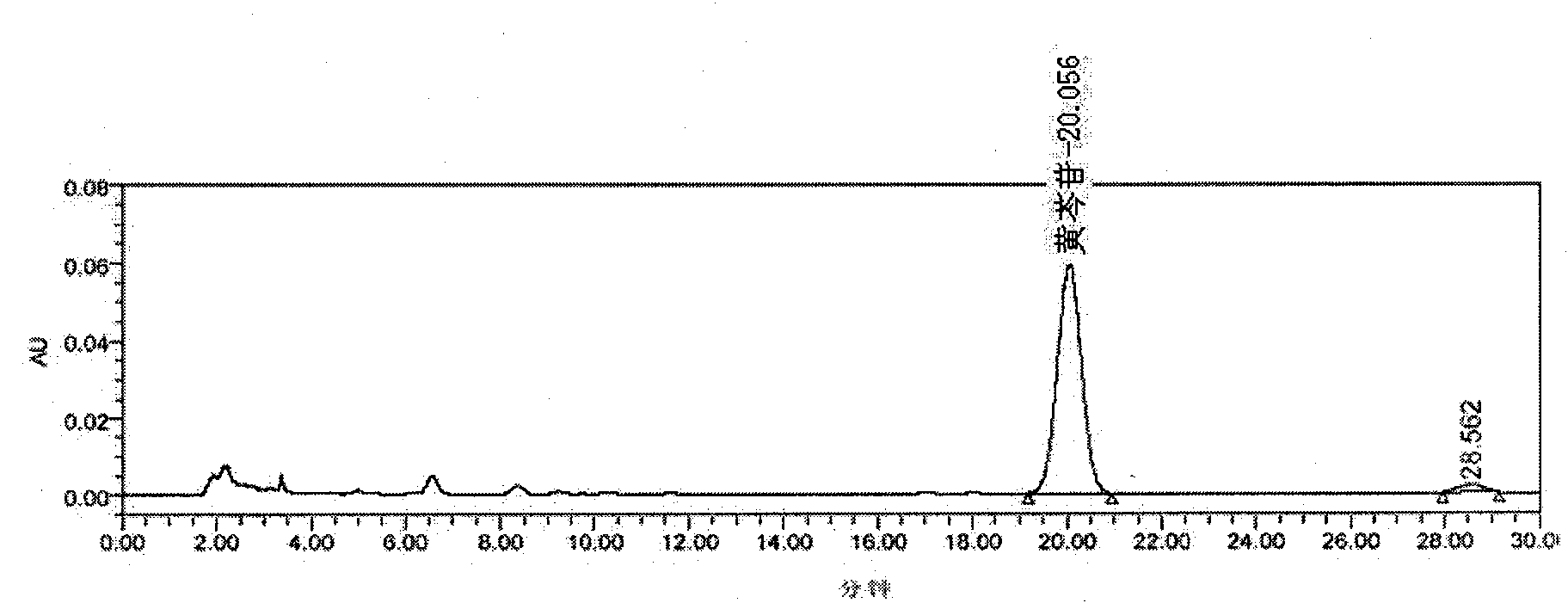
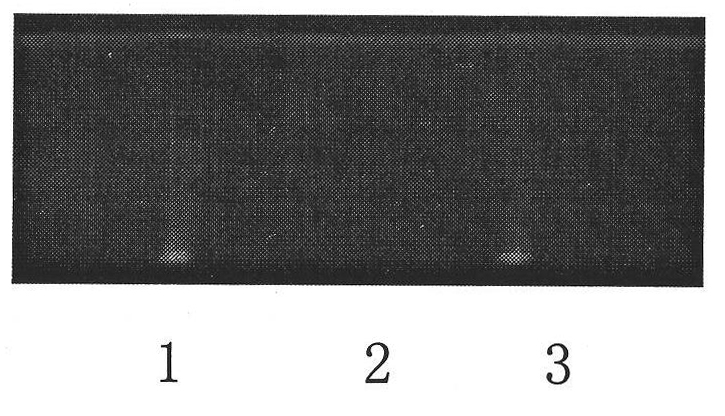
[0048] In the original quality standard of Qingre Liangxue Pills, there was the identification of Scutellaria baicalensis, but there was no content determination item. This time, the TLC identification of Rehmannia glutinosa and the content determination of Scutellaria baicalensis were added to the standard. At the same time, the identification method of scutellaria baicalensis in the original standard was revised again.
[0049] 1. Identification of Scutellaria baicalensis
[0050] 1.1 Materials and reagents
[0051] Methanol, ethyl acetate, butanone, formic acid: analytically pure
[0052] Baicalin reference substance: National Institute for the Control of Pharmaceutical and Biological Products, batch number: 110715-200514
[0053] Sample of Qingreliangxue Pill: produced by Lerentang Pharmaceutical Factory of Tianjin Zhongxin Pharmaceutical Group Co., Ltd.;
[0054] Batch number: D081001
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com