Method for treating odorous gas by utilizing solution of hexavalent ferric salt
A solution treatment, high iron salt technology, applied in separation methods, chemical instruments and methods, dispersed particle separation, etc., can solve the problems of long removal of odor, generation of treatment time, etc., and achieve good stability, long-lasting strong oxidation, high efficiency and stability Effect of removing bad smell gas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of hexavalent ferric salt solution
[0023] The hexavalent ferric salt was prepared as follows: 53.4 g KMnO 4 Added to 330 ml of 37% hydrochloric acid by volume to generate Cl 2 . Cl 2 Pass into the KOH solution that has been prepared and cooled in advance, and stir for more than two hours. Another 180 g of KOH was added to the solution, and the solution was cooled. The precipitated KCl was removed from the solution by filtration with GF / C filter paper, leaving a concentrated, strongly basic KClO yellow filtrate. 75 g of powdered Fe(NO 3 ) 3 9H 2 O was slowly added to the KClO filtrate with rapid stirring. Then 60 g KOH was added to the above solution, and the mixture was stirred for 20 minutes. The solution was allowed to stand for 40 minutes, and the resulting deep purple slurry was filtered through a glass filter, and the filtrate was discarded. Wash the precipitate with pre-cooled 1 mol / L KOH solution. The rinsed filtrate was collected and add...
Embodiment 2
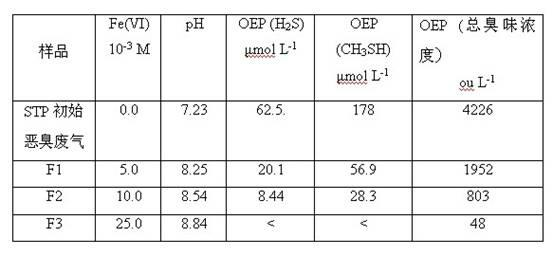
[0026] Hexavalent ferric salt oxidation technology to treat H 2 S malodorous gas
[0027] The series of experiments were performed at H 2 The concentration of S gas is 50 ppm / v, and the Fe(VI) solution prepared by using different pH buffer solutions is sprayed out from the nozzle at the upper end of the purification equipment. The molar concentration range of ferrate was between 0.08-1.39 mM. At pH 7.0, when ferrate and H 2 The molar ratio of S ([Fe(VI)] / [H 2 S]) are 0.58, 0.83, 1.66, 2.49, 2.77, 3.00 and 4.15 respectively, H 2 S is converted to SO 4 2- The efficiencies were 26.4%, 35.0%, 68.6%, 94.7%, 98.0%, 99.3% and 100%, respectively. and OH is released during the reaction - ions, the pH of the mixed solution increased from the initial 8.4 to 9.5. At pH 9.0, when [Fe(VI)] / [H 2When the molar ratio of S] is 0.56, 0.84, 1.10, 1.68, 2.20, 2.52, 2.80 and 4.20, the H 2 S is converted to SO 4 2- The efficiencies were 37.9%, 44.5%, 58.5%, 66.9%, 87.2%, 96.2%, 97.9, an...
Embodiment 3
[0031] Treatment of CH by Hexavalent Ferric Salt Oxidation Technology 3 SH malodorous gas
[0032] The series of experiments were performed at CH 3 The concentration of SH gas is 50 ppm / v, and the Fe(VI) solution prepared by buffer solution is sprayed from the upper nozzle of the purification equipment. The molar concentration of ferrate ranges from 0.35 mM to 1.5 mM. At pH 9.0, when ferrate and CH 3 The molar ratio of SH ([Fe(VI)] / [CH 3 SH]) are 2.3, 3.0, 4.0, 4.6, 5.0, 6.0 and 8.0 respectively, the CH 3 The SH removal efficiencies were 14.5%, 34.3%, 60.3%, 95.0%, 97.3%, 99.0% and 100%, respectively. OH is released during the reaction - ions, the pH of the mixed solution increased from the initial 8.14 to 9.44.
[0033] Ferrate and CH 3 The stoichiometric equation for SH is as follows:
[0034] 14HFeO 4 - + 3CH 3 SH+9H 2 O → 14Fe(OH) 3 + 3SO 4 2- + 3CO 3 2- + 2OH - (II)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com