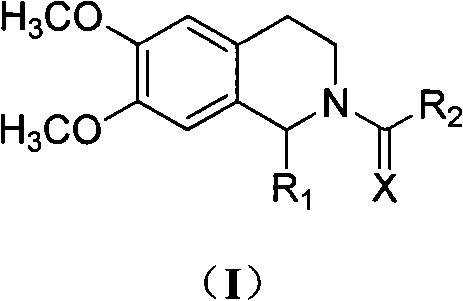
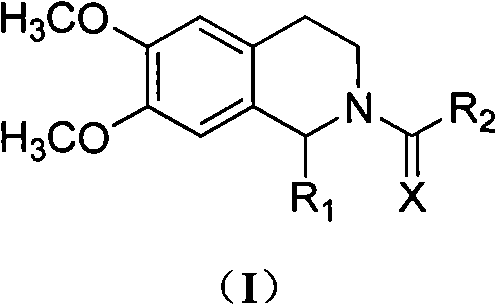
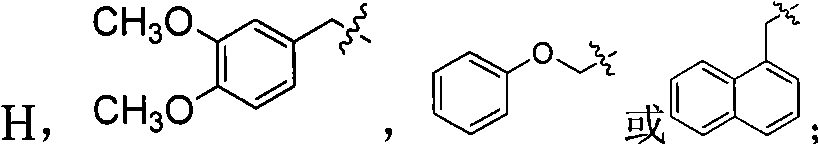
Tetrahydroisoquinoline derivatives and preparation method and application thereof
A technology of tetrahydroisoquinoline and its derivatives, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc. It can solve the problems of low reversal activity, high fat solubility, and weak specificity of action.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1: 6,7-dimethoxy-3,4-dihydroisoquinoline (4a)
[0126] 40g (0.22mol) 3,4-dimethoxyphenethylamine (compound 2) mixed with 18.4g 36% (0.22mol) formaldehyde, gradually warming up to 100 ° C, reflux for 30 minutes, stop heating, after a little cooling (yellow oil-water mixture) suction pipette to remove the upper aqueous solution, then add 4 times the amount of 23% hydrochloric acid, evaporate the reaction solution to dryness to obtain a brownish yellow solid, recrystallize with 200ml of 95% ethanol to obtain 35g of white needle crystals, add the crystals to Concentrated ammonia water 45ml, slightly heated until completely dissolved without viscous matter, extracted with dichloromethane, washed with saturated brine until neutral, dried over anhydrous sodium sulfate, filtered and evaporated to dryness to obtain 28.5g of yellow solid powder, yield: 67.9%, used directly in the next reaction.
Embodiment 2
[0127] Example 2: 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (5a)
[0128] 28.5g (0.15mol) of compound 4a was dissolved in 160ml of methanol, 16g (0.38mol) of potassium borohydride was added in batches under an ice-water bath, and stirred at room temperature for 22h. The reaction solution was slowly poured into 500ml of ice-cold brine, and allowed to stand overnight to fully hydrolyze the potassium borohydride. Extracted with dichloromethane (100ml×3), washed with saturated brine until neutral, dried over anhydrous sodium sulfate, filtered and evaporated to dryness to obtain 26.1g of white solid 5a, yield: 92%. mp: 74-76°C.
Embodiment 3
[0129] Example 3: N-(3,4-dimethoxy)phenethyl-(3,4-dimethoxy)phenylacetamide (3b)
[0130] 3,4-dimethoxyphenylethylamine (dimethoxyphenethylamine (distilled under reduced pressure, 140-170 / 1mmHg cut) 19.7g (0.106mol) is mixed with 3,4-dimethoxyphenylacetic acid 20.0g (0.102mol), pass Nitrogen, slowly heated to 190 ° C, water vapor is generated, all solids melt, keep warm for 3 hours, let cool to room temperature, add 150ml of chloroform to dissolve, and wash the chloroform solution with 3% hydrochloric acid, clear water, 3% NaOH aqueous solution, and saturated saline successively until Neutral, anhydrous Na 2 SO 4 Dry, evaporate to near dryness under reduced pressure, cool slightly, add 100ml of diethyl ether, shake gently, precipitate white solid, filter to obtain 32g of white solid, yield: 87%, mp: 121-123°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com