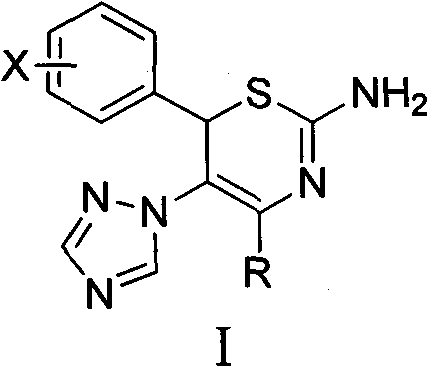
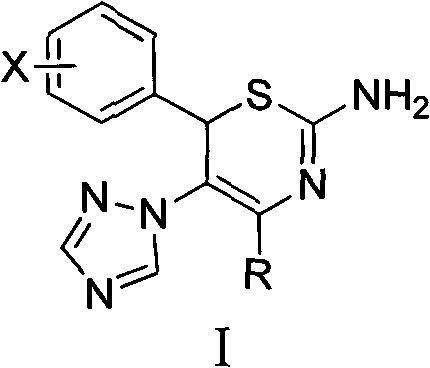
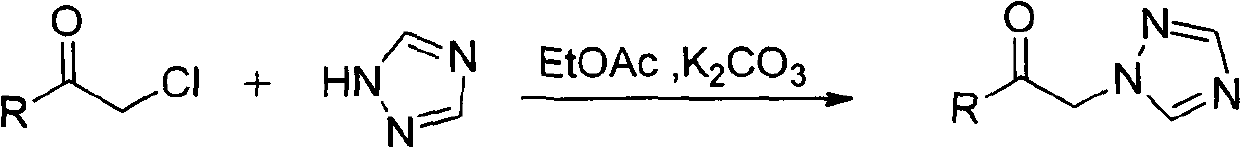4-alkyl-6-aryl-5-(1, 2, 4-triazole-1-yl)-2-amino-1, 3-thiazine and application thereof
An alkyl and aryl technology, applied in the field of new compounds and their preparation, can solve the problems of no reports of insecticidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0040] Preparation of Example 14-tert-butyl-6-(4-methoxyphenyl)-5-(1,2,4-triazol-1-yl)-2-amino-1,3-thiazine
[0041] (1) Preparation of 3,3-dimethyl-1-(1,2,4-triazol-1-yl)-2-butanone
[0042] Chloropinatone (0.05mol), 1,2,4-triazole (0.05mmol), potassium carbonate (0.10mol), catalytic amount PEG600, ethyl acetate (60mL) were stirred and refluxed, reacted for 5.0h, filtered, and Add 60% HNO dropwise to the filtrate 3 (0.05mol) into a salt, filtered, the filter cake was placed in ethyl acetate, ammonia water was added dropwise, the solid was dissolved, separated, dried, and the solvent was recovered by distillation to obtain 3,3-dimethyl-1-(1,2, 4-triazol-1-yl)-2-butanone; yield 85.6%, melting point 60-62°C.
[0043] (2) Preparation of 4,4-dimethyl-1-(4-methoxyphenyl)-2-(1,2,4-triazol-1-yl)-1-penten-3-one
[0044] 3,3-Dimethyl-1-(1,2,4-triazol-1-yl)-2-butanone (0.011mol), piperidine (0.1mL), toluene (30mL), add dropwise 4- Methoxybenzaldehyde (0.010mol) toluene solution (20...
Embodiment 24
[0047] Example 24- Preparation of tert-butyl-6-(4-chlorophenyl)-5-(1,2,4-triazol-1-yl)-2-amino-1,3-thiazine hydrochloride
[0048] (1) Preparation of 4,4-dimethyl-1-(4-chlorophenyl)-2-(1,2,4-triazol-1-yl)-1-penten-3-one
[0049] 3,3-Dimethyl-1-(1,2,4-triazol-1-yl)-2-butanone (0.011mol), piperidine (0.1mL), toluene (30mL), add dropwise 4- Chlorobenzaldehyde (0.010mol) toluene solution (20mL), stirred and refluxed, reacted for 8h, the reaction solution was washed with 3 × 50mL water, separated and distilled to recover the solvent to obtain yellow viscous 4,4-dimethyl-1-( 4-chlorophenyl)-2-(1,2,4-triazol-1-yl)-1-penten-3-one.
[0050] (2) 4-tert-butyl-6-(4-chlorophenyl)-5-(1,2,4-triazol-1-yl)-2-amino-1,3-thiazine hydrochloride to prepare
[0051] 4,4-Dimethyl-1-(4-chlorophenyl)-2-(1,2,4-triazol-1-yl)-1-penten-3-one, thiourea (0.011mol) , ethanol (60ml), stirred and refluxed, and reacted for 6h. Cooled, precipitated solid, filtered and dried to obtain 4-tert-butyl-6-(4-chlor...
Embodiment 34
[0052] Example 34- Preparation of tert-butyl-6-(2-methoxyphenyl)-5-(1,2,4-triazol-1-yl)-2-amino-1,3-thiazine
[0053] (1) Preparation of 4,4-dimethyl-1-(2-methoxyphenyl)-2-(1,2,4-triazol-1-yl)-1-penten-3-one
[0054] 3,3-Dimethyl-1-(1,2,4-triazol-1-yl)-2-butanone (0.011mol), piperidine (0.1mL), toluene (30mL), add dropwise 4- Chlorobenzaldehyde (0.010mol) toluene solution (20mL), stirred and refluxed, reacted for 7h, the reaction solution was washed with 3×50mL water, separated, dried, and the solvent was recovered by distillation to obtain a yellow sticky substance 4,4-dimethyl-1 -(2-methoxyphenyl)-2-(1,2,4-triazol-1-yl)-1-penten-3-one.
[0055] (2) Preparation of 4-tert-butyl-6-(2-methoxyphenyl)-5-(1,2,4-triazol-1-yl)-2-amino-1,3-thiazine
[0056] 4,4-Dimethyl-1-(2-methoxyphenyl)-2-(1,2,4-triazol-1-yl)-1-penten-3-one, thiourea (0.011 mol), ethanol (60ml), stirred and refluxed, reacted for 24h, added ammonia water for neutralization, vacuum distillation and column chroma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com