Fuel cell catalyst taking conductive ceramic boron carbide as supporter and preparation method thereof
A technology of fuel cells and conductive ceramics, applied in the direction of physical/chemical process catalysts, catalyst carriers, chemical instruments and methods, etc., can solve the problems that have not yet been reported on proton exchange membrane fuel cell catalysts, achieve high methanol formic acid oxidation ability, synthesis The process is simple, the effect of wear-resistant and conductive properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
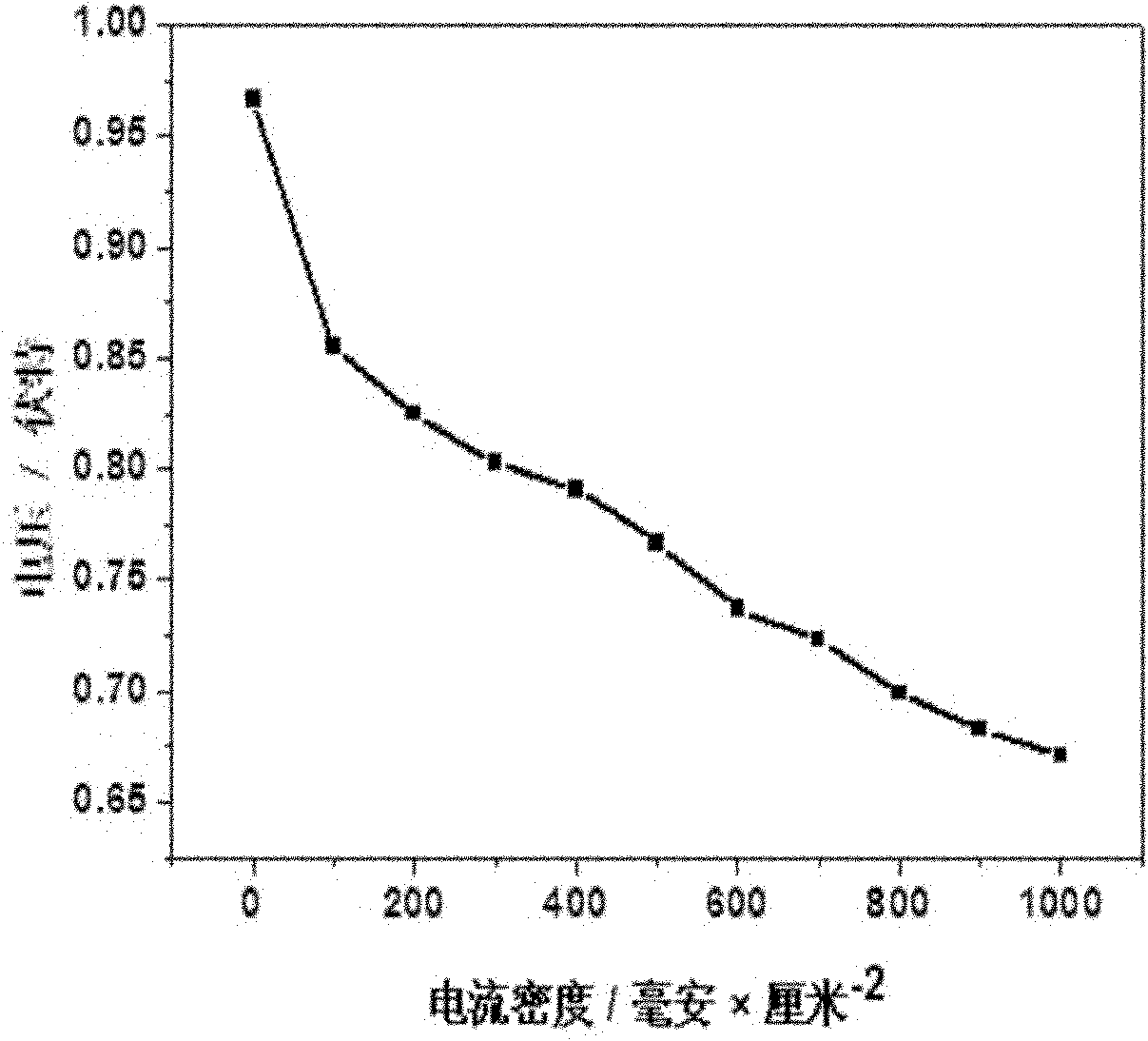
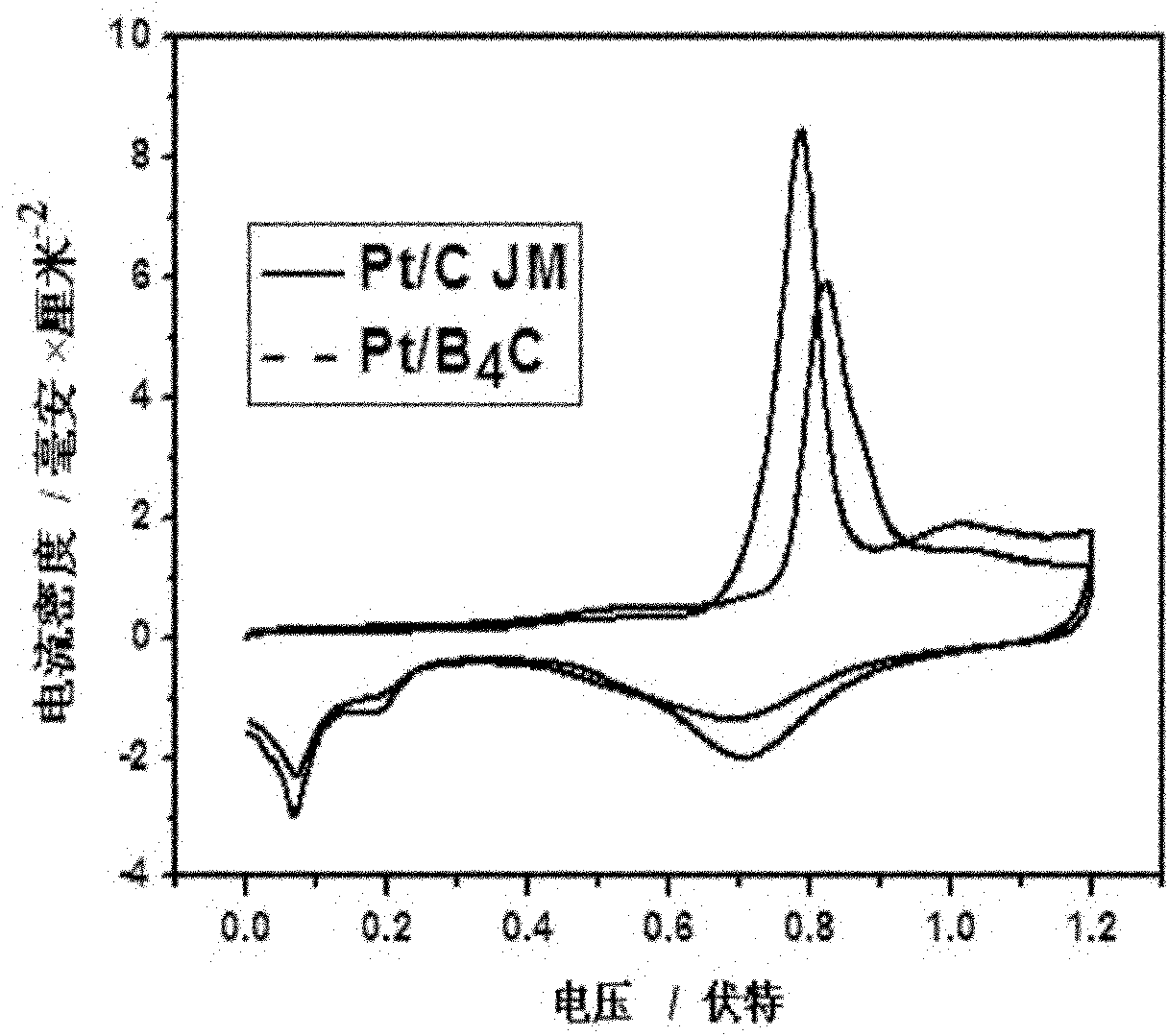
[0035] Mix 150 mL of pure ethylene glycol solution with 50 mL of 1.4 mg / mL HO 2 PtCl 6 ·6H 2 A solution mixed in O, in N 2Stir well under protection to form a uniform mixed solution, then add 2 mol / liter sodium hydroxide solution drop by drop to adjust the pH value of the mixed solution to 9-13, then heat the mixed solution to 130-160°C in an oil bath, and condense After refluxing for 2 to 5 hours, the color of the solution gradually changed from light yellow to dark brown, and a stable Pt colloid was obtained. Weigh 280 mg of boron carbide powder with an average particle size of 50 nanometers, add it to 100 ml of ethylene glycol solution for ultrasonic dispersion for 30-60 minutes, then add it to the above stable Pt colloid solution, stir for 8-10 minutes Hour. After filtering and washing with aqueous alcohol solution, Pt / B 4 C catalyst. Compared with the carbon support catalyst Pt / C, the active area is increased by 30%, the oxidation potential of carbon monoxide is red...
Embodiment 2
[0039] Get 280 mg of boron carbide powder with an average particle diameter of 50 nanometers, disperse it into 100 ml of ethylene glycol solution and ultrasonically disperse it for 30 to 60 minutes to prepare a boron carbide solution for use. Mix 150 ml of ethylene glycol solution with 50 ml 1.4 mg / ml H 2 PtCl 6 ·6H 2 The solution of O is mixed, and the above boron carbide solution is mixed with H 2 PtCl 6 ·6H 2 A solution mixed in O, in N 2 Stir under protection for 10-20 minutes to form a uniform mixed solution, then add 2 mol / L NaOH solution drop by drop to adjust the pH value of the mixed solution to 9-13, and then heat the mixed solution to 130-160°C in an oil bath, Condensate and reflux for 2-5 hours, stop heating and continue stirring for 8-10 hours. After filtering and washing with alcohol aqueous solution, Pt / B 4 C catalyst. Compared with the traditional carbon support Pt / C catalyst, the active area is increased by 21%, the carbon monoxide oxidation potential ...
Embodiment 3
[0041] Take 1.4 mg / ml PdCl in concentrated hydrochloric acid 2 Mix 50 ml of the solution with 150 ml of ethylene glycol in N 2 Stir under protection for 10-20 minutes to form a uniform mixed solution, and then dropwise add 2 mol / liter sodium hydroxide solution to adjust the pH value of the mixed solution to 8-9, and weigh 280 mg of Boron carbide powder is added to 100 ml of ethylene glycol solution and ultrasonically dispersed for 30 to 60 minutes, and it is mixed with PdCl 2 Mix the solutions, reflux at 80-90°C for 2-3 hours, stop heating and continue stirring for 8-10 hours, filter and wash with alcohol aqueous solution to obtain Pd / B 4 C catalyst. Compared with the traditional carbon-supported Pd / C catalyst, the active area is increased by 26%, the carbon monoxide oxidation potential is reduced by 0.043 volts, and the peak value of the oxidation current is increased by 17%.
[0042] Preparation of fuel cell chip CCM: add the prepared catalyst to deionized water and 5% pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com