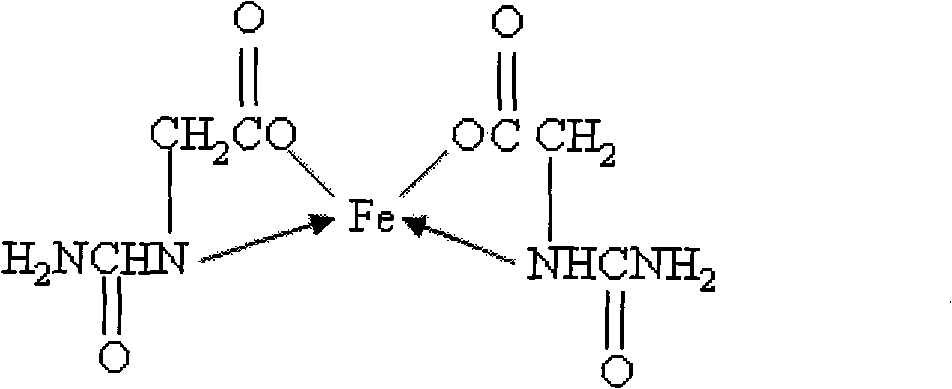
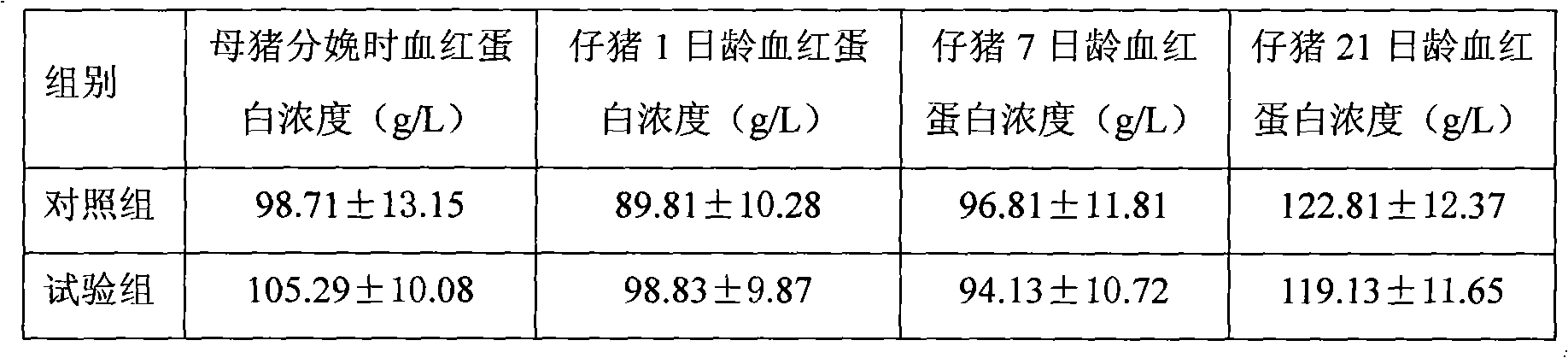
Dipeptide iron chelate for feed additives and preparation method thereof
A technology for chelating iron and carbamoylglycine dipeptide, which is applied in dipeptide, animal feed, animal feed, etc., can solve the problem of poor stability of chelated iron, achieve low price, easy access to raw materials, and high absorption rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment one, the preparation of dipeptide chelated iron
[0029] Step 1, the preparation of carbamoylglycine dipeptide:
[0030] Mix 100 grams of glycine and 60 grams of potassium cyanate, add 200 ml of water and 4 grams of potassium hydroxide, react at room temperature for 20 hours, adjust the pH to 4.5 with hydrochloric acid, crystallize at 0 ° C, filter and dry to obtain carbamoylglycine Dipeptide 115 grams. Step 2, the preparation of carbamoylglycine dipeptide chelated iron:
[0031]Take 100 grams of carbamoylglycine dipeptide, 50 grams of ferrous sulfate, add 200 milliliters of water, adjust pH=6 with 1.0 mol / L sodium hydroxide, heat on 65 °C water solution for 4 hours, filter the insoluble matter, and the filtrate Add 95% ethanol, precipitate out, and dry to obtain 65 grams of carbamoylglycine dipeptide chelated iron.
[0032] The valence state of iron element detected by potassium permanganate titration method or similar method is +2, the yield of carbamoyl...
Embodiment 2
[0033] Embodiment two, the preparation of dipeptide chelated iron
[0034] Step 1, the preparation of carbamoylglycine dipeptide
[0035] Mix 110 grams of glycine and 70 grams of potassium cyanate, add 210 milliliters of water, and add 5 grams of potassium hydroxide, react at room temperature for 21 hours, adjust to pH = 4.5 with hydrochloric acid, crystallize at 1 ° C, and filter to obtain carbamoglycine dipeptide 135 grams. Step 2, preparation of carbamoylglycine dipeptide chelated iron
[0036] Take 110 grams of carbamoylglycine dipeptide, 60 grams of ferrous sulfate, add 220 milliliters of water, use 1.5 mol / =sodium hydroxide, adjust pH=6.2, heat on 70 ° C water solution for 4.5 hours, filter the insoluble matter, add the filtrate 95% ethanol, precipitated, and dried to obtain 63.8 grams of carbamoylglycine dipeptide chelated iron.
[0037] Potassium permanganate titration method or similar method is used to detect the valence state of iron element as +2 valence, the yi...
Embodiment 3
[0038] Embodiment three, the preparation of dipeptide chelated iron
[0039] Step 1, the preparation of carbamoylglycine dipeptide
[0040] Mix 120 grams of glycine and 80 grams of potassium cyanate, add 215 milliliters of water and 5.5 grams of potassium hydroxide, react at room temperature for 22 hours, adjust to pH = 4.5 with hydrochloric acid, crystallize at 2°C, and filter to obtain carbamoylglycine dipeptide 145 grams. Step 2, preparation of carbamoylglycine dipeptide chelated iron
[0041] Take 120 grams of carbamoylglycinyl nitrogen dipeptide, 65 grams of ferrous sulfate, add 240 milliliters of water, use 2.0 mol / L sodium hydroxide to adjust the pH=6.8, heat it in water at 75 ° C for 5 hours, filter the insoluble matter, Add 95% ethanol to the filtrate, precipitate out, and dry to obtain 72 grams of carbamoylglycine dipeptide chelated iron.
[0042] The valence state of iron element detected by potassium permanganate titration method or similar method is +2, the yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com