Experiment simulation system for exploiting natural gas hydrate by using CO2
An experimental simulation and hydrate technology, which is applied in the fields of fluid extraction, earth-moving drilling, climate sustainability, etc., and can solve problems such as few devices and inability to meet research needs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
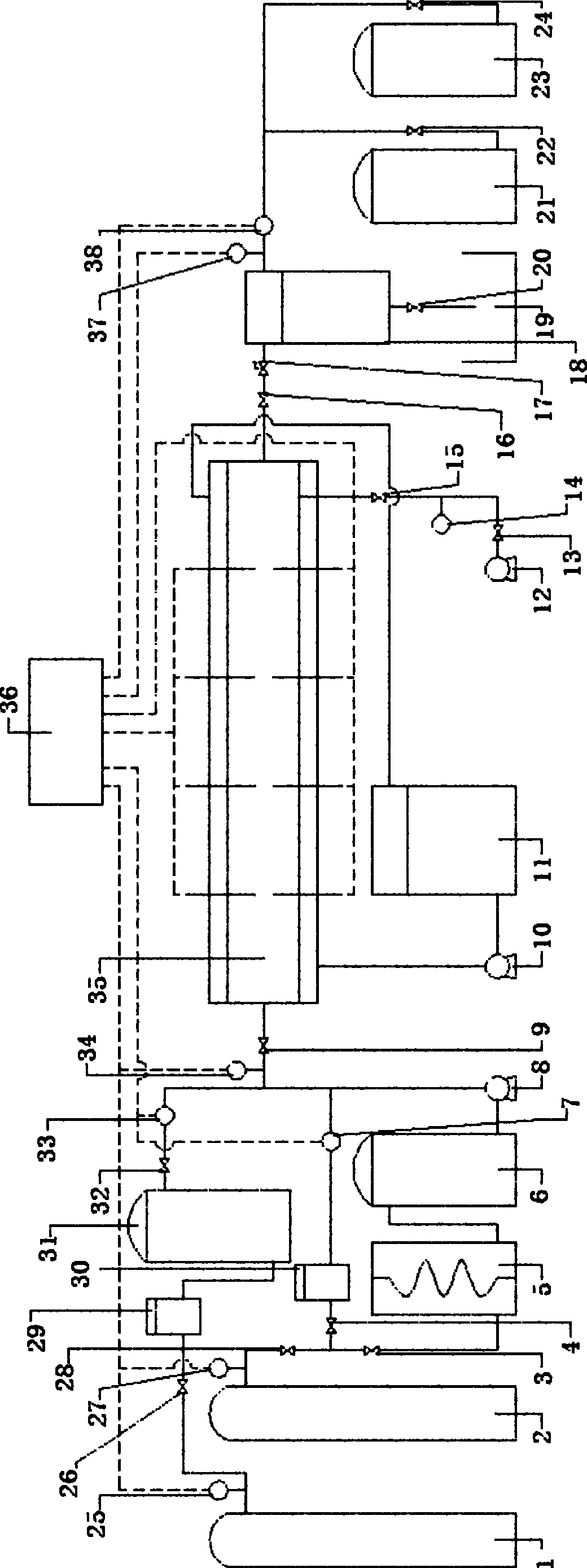
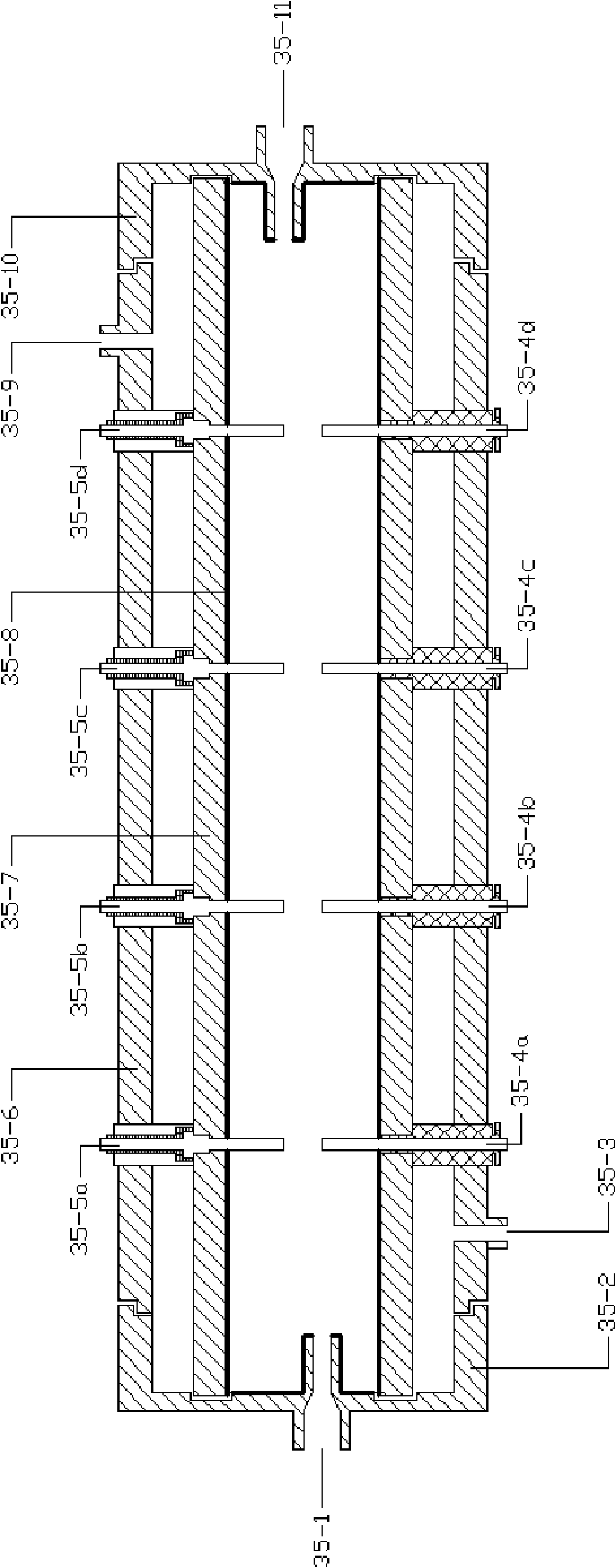
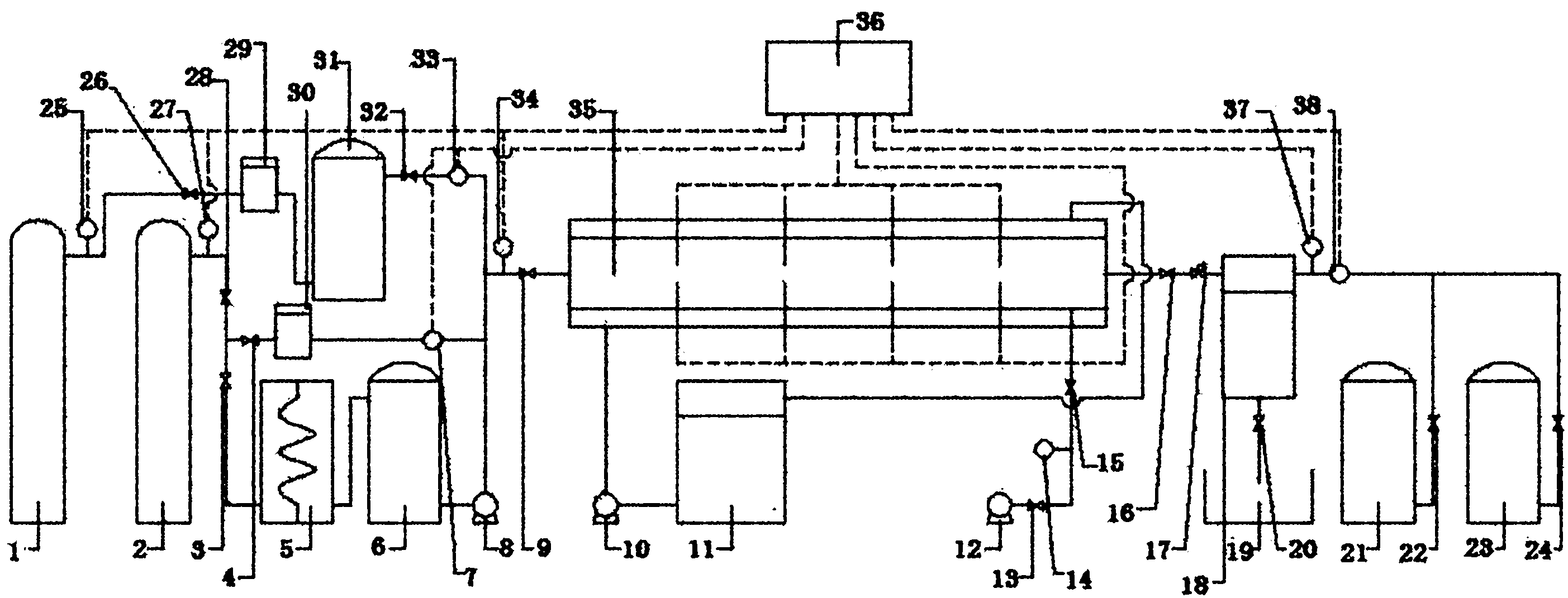
[0031] see figure 1 As shown, a CO 2 The experimental simulation system for natural gas hydrate exploitation includes CH4 supply system, CO 2 Five systems including supply system, hydrate reactor 35, refrigeration system, gas recovery system and data acquisition system:
[0032] The CH4 supply system mainly includes sequentially connected CH 4 A gas cylinder 1, a pressure sensor 25, a pressure regulator 29, a buffer tank 31, a mass flow meter 33, and a hydrate reactor 35 are connected. CH 4 Gas cylinder 1 provides CH for the hydrate production in the early stage of the experiment 4 Air source, pressure regulator 29 will come from CH 4 The gas source pressure of the gas cylinder 1 is adjusted to an experimental set value, the function of the buffer tank 30 is to stabilize the pressure, and the pressure sensor 25 measures CH 4 The pressure of the supply system, the mass flow meter 33 metering experiment consumption CH 4 gas flow. So CH 4 The main function of the supply ...
example 1
[0043] Experimental simulation of liquid CO 2 Replacement of CH in hydrate 4 .
[0044] Sieve hundreds of grams of 20-40 mesh quartz sand with a standard sieve, wash and air dry for later use. It is determined that the porosity of the quartz sand is 46%, and the moisture content after natural air drying is 5%. When the moisture content is 30% of the weight of the quartz sand, the quartz sand is saturated. In this experiment, 290g of adaptive sand (14.5g of water in it) was taken, and 14g of 290ppm SDS aqueous solution was added thereto. The total water content of the quartz sand was 28.5g, and the saturation humidity was 32%.
[0045] Put the above-mentioned hydrated quartz sand into the hydrate reactor 33 (basically filled), vacuumize the system with the vacuum pump 11, and then open the CH 4 Supply system, use mass flow meter 30 to measure and feed 559mmolCH4 gas, at this time the gas CH in the hydrate reactor 4 The initial pressure of the hydrate reactor reaches 12-15MP...
example 2
[0049] Experimental simulation of gaseous CO 2 Replacement of CH in hydrate 4 .
[0050] CH in this experiment 4 The preparation method of hydrate is exactly the same as the method of liquid replacement, and will not be repeated here. in CH 4 After hydrate formation, in order to quantitatively understand the CH formed in quartz sand 4 For the amount of hydrate, adjust the refrigerator 10 to reduce the temperature of the reaction system to 271.2K 1 hour before the hydrate formation reaction has been completed and the subsequent replacement reaction starts. At this time, CH 4 Hydrate has a self-protection effect, and CO 2 Displace free CH 4 . CH formed at this time 4 CH in hydrate 4 The content is 242.7 mmol. To understand the gaseous CO 2 Replacement of CH in hydrate 4 Kinetic performance, the pressure in the reactor is determined to be 2.5MPa with the back pressure 16, and the temperature in the reactor is controlled to be 277.7±0.2K by the refrigerator 10. Due to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com