Preparation method and product of ferric phosphate
A technology of iron phosphate and phosphoric acid, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of high production cost, high acid ion content, complex process, etc., and achieve low production cost, cost saving, Equipment simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
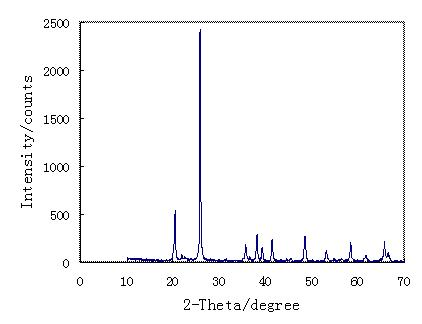
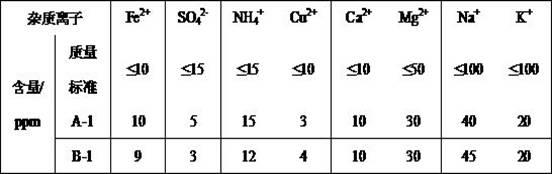
Image
Examples
Embodiment 1
[0027] 1. Ferrous oxalate (FeC 2 o 4 2H 2 O) 180g was dissolved in 1.5 L of deionized water to make a slurry. After adding 85% phosphoric acid (concentrated phosphoric acid) according to the stoichiometric ratio of 1:1, stir for 30 minutes to make it evenly mixed.
[0028] 2. Add 10% excess hydrogen peroxide solution (concentration: 30%) according to the stoichiometric ratio to the above-mentioned homogeneously mixed slurry, control the adding speed, so as to prevent the solution from splashing out of the beaker during the reaction, and stir with a magnetic stirrer at the same time, Continue to stir for 60 minutes after the addition of hydrogen peroxide is complete, until the ferrous iron is completely converted to ferric iron, and the slurry becomes a clear solution.
[0029] 3. Vacuum filter the above solution to obtain the filtrate. At room temperature (20°C), stir the solution with a magnetic force, and at the same time slowly add 0.1 mol / L ammonia water to the soluti...
Embodiment 2
[0033] 1. Ferrous oxalate (FeC 2 o 4 2H 2 (0) 360g was dissolved in 0.54 L of deionized water to make slurry, according to the stoichiometric 1:1. After adding 85% phosphoric acid, stirred for 60 minutes to make it evenly mixed;
[0034] 2. According to the operation method described in Example 1, an excess of 20% hydrogen peroxide solution (concentration 30%) was added to the uniformly mixed slurry, and stirring was continued for 30 minutes after the hydrogen peroxide was added until the ferrous iron was completely converted into trivalent iron. Valence iron until the slurry turns into a clear solution.
[0035] 3. After the above solution was vacuum filtered to obtain the filtered solution, the solution was heated to 80°C, stirred with a magnetic stirrer, and at the same time, 0.5 mol / L ammonia water was slowly added to the solution, and the ammonia water addition rate was controlled to be 5ml / min. until the pH of the solution was adjusted to 2.0.
[0036] 4. After a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com