Preparation method of atorvastatin calcium isomer mixture and its intermediate
A technology for isomer mixtures and compounds, which is applied in the field of chemical synthesis and can solve the problems of complex quality monitoring methods and no diastereomer synthesis and analysis involved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
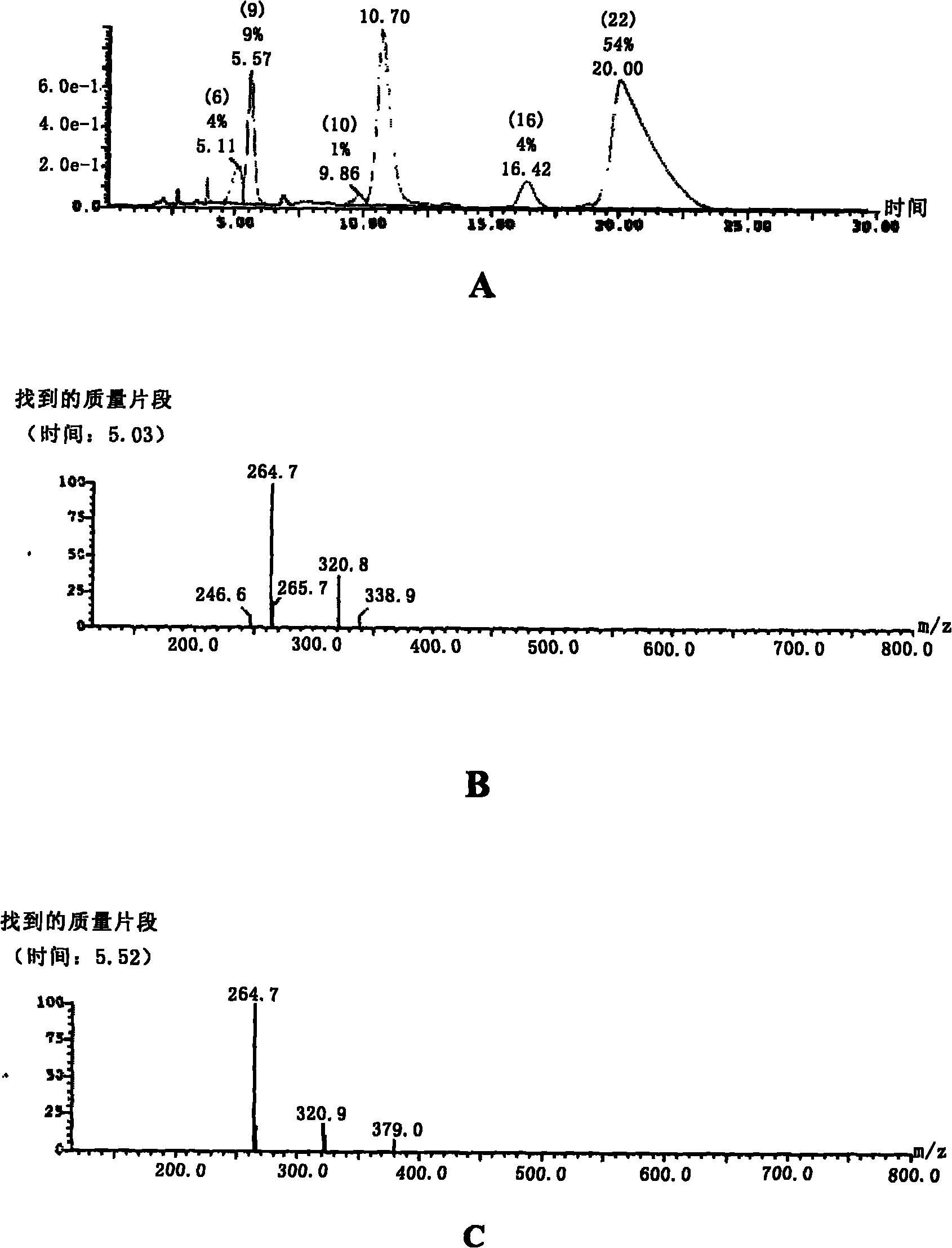
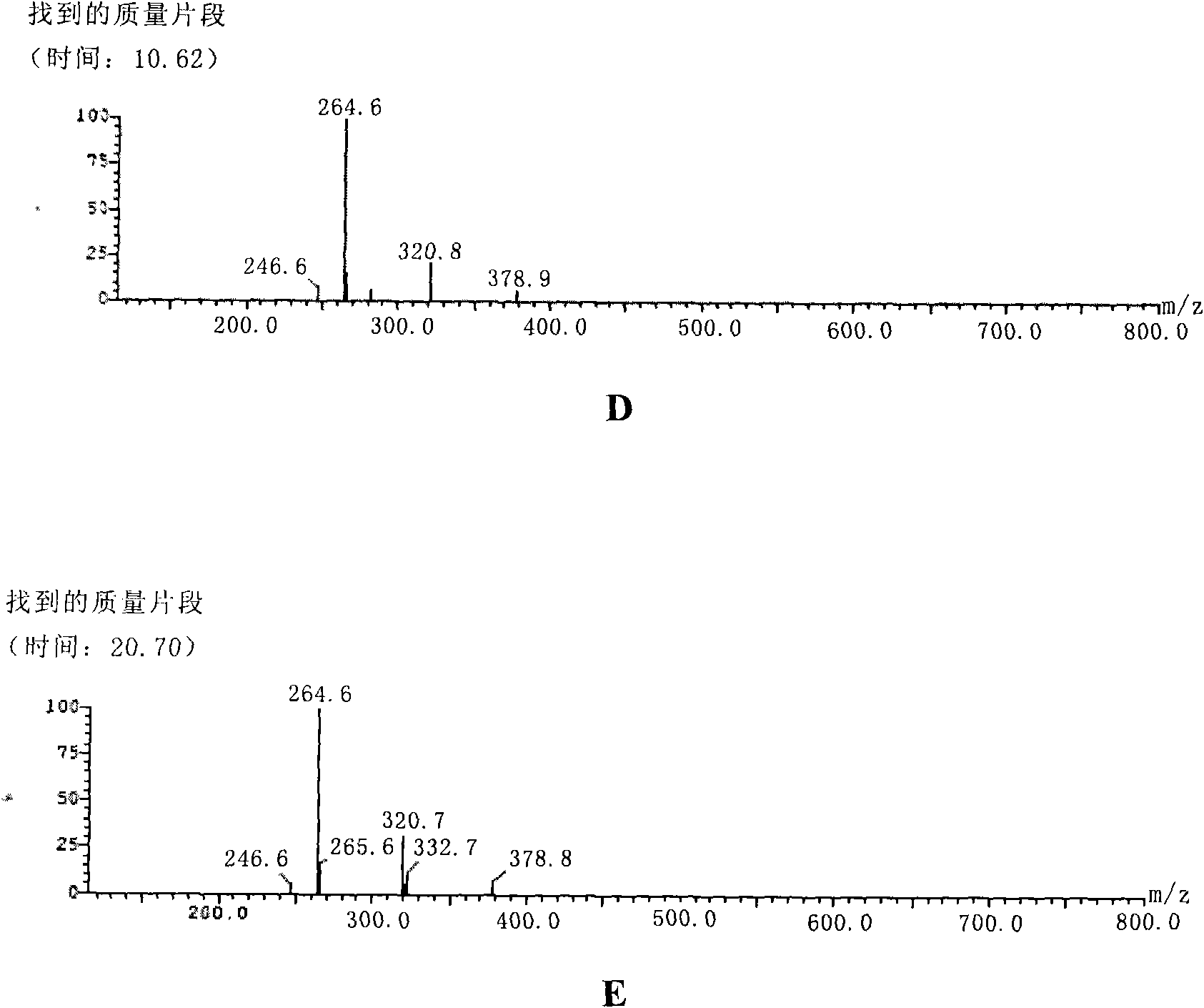
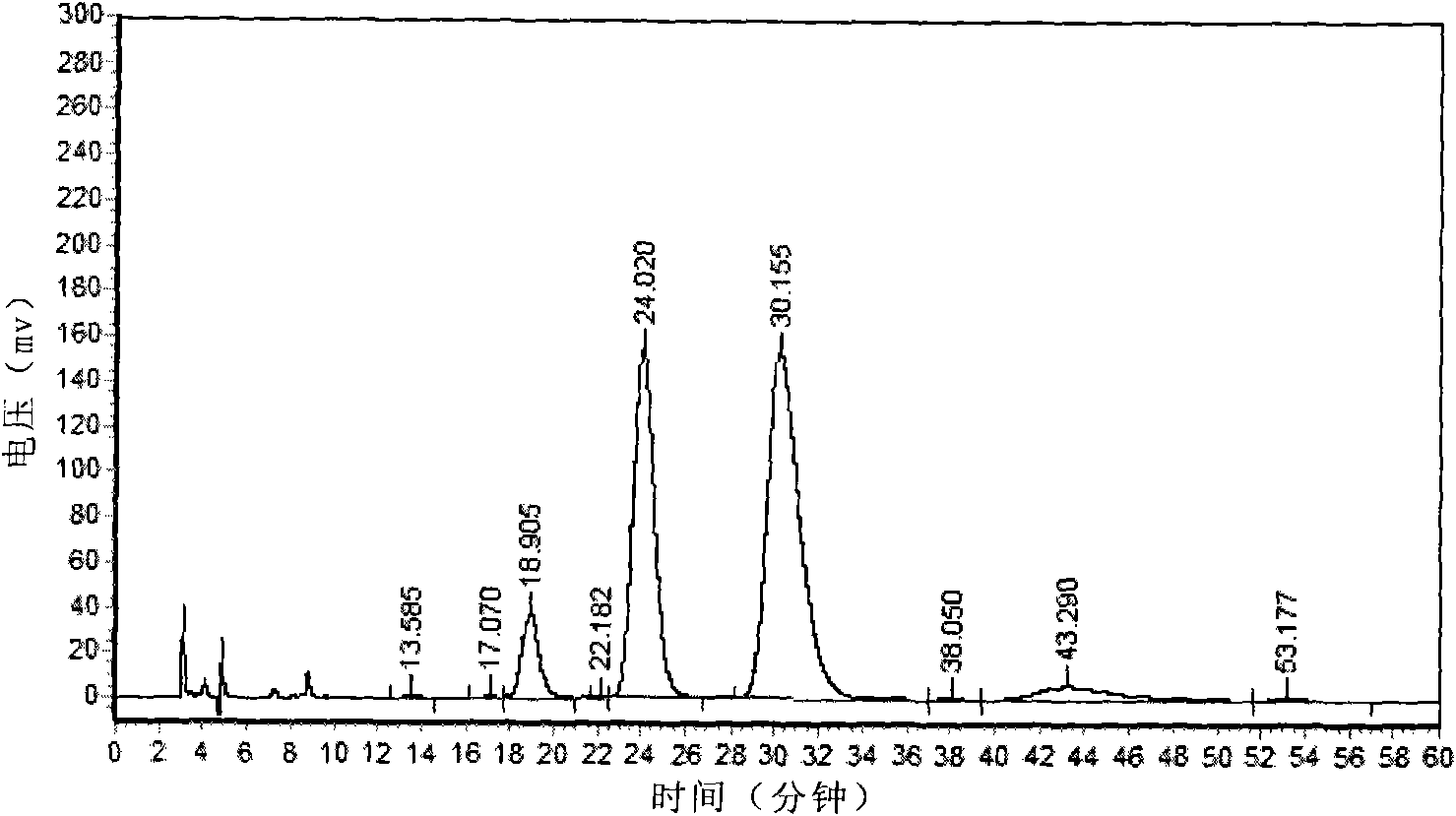
Image
Examples
preparation example Construction
[0074] The present invention provides a method for preparing the isomer mixture shown in formula 11A, the method comprising the steps of:
[0075] (a) mixing the compound shown in formula 2 and compound RCOX to obtain the compound shown in formula 7A;
[0076] (b) ring-opening the compound shown in Formula 7A in the presence of an acid to obtain a compound shown in Formula 8A;
[0077] (c) mixing the compound shown in formula 8A with an oxidizing agent to obtain a mixture of isomers shown in formula 9A;
[0078] (d) reducing the compound shown in formula 9A to obtain a mixture of isomers shown in formula 10A; and
[0079] (e) mixing the isomer mixture of the compound shown in formula 10A, the catalyst, acetone, and 2,2-dimethoxypropane to obtain the isomer mixture shown in formula 11A;
[0080] Wherein R is an ultraviolet emitting group, an aryl group; an aryloxy group;
[0081] X is fluorine, chlorine, bromine, or iodine.
[0082]
[0083] In a preferred example of the...
Embodiment 1
[0152] Compound 2 was reacted with benzoyl chloride to obtain compound 3, the molar ratio of compound 2 and benzoyl chloride was 1:1, 2 (10g, 0.037mol) was dissolved in dichloromethane, and benzoyl chloride (5.18g, 0.037mol) was added dropwise ), control the temperature not to exceed 40°C, and stir at 30°C for 2h after the dropwise addition is completed. Water was added for washing, and the layers were separated. The organic phase was dried over anhydrous sodium sulfate and then concentrated to obtain 10.1 g of crude compound 3 with a yield of 72%.
Embodiment 2
[0154] Dissolve compound 3 (10.1g, 0.027mol) in methanol, add dropwise 10% hydrochloric acid, the molar ratio of 10% hydrochloric acid and compound 3 is 2:1, stir for 2-3h after the dropwise addition, add saturated sodium bicarbonate solution to adjust When the pH reached 6-7, concentrated, dichloromethane was added to separate the layers, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 8.5 g of crude isomer mixture 4 with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com