Red sage root extract sustained-release micropills and preparation method and application thereof
A technology of sustained-release pellets and extracts, applied in drug combinations, pharmaceutical formulas, plant raw materials, etc., can solve problems such as difficult research and complex mechanism of action, and achieve the effects of expanding peripheral blood vessels, stable release, and improved blood circulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Prescription
[0037] 1.1 Pill Core Prescription
[0038] Danshen Extract 150g
[0039] 650g microcrystalline cellulose
[0040] Starch 150g
[0041]Croscarmellose Sodium 25g
[0042] Sodium carboxymethyl starch 25g
[0043] 1.2 Prescription of coating solution
[0044] Ultrafine Talc Powder 1.5g
[0045] 37.5 grams of water
[0046] Copolymer of methyl methacrylate and ethyl acrylate (trade name NE-30D )
[0047] 75.0 grams
[0048] 2. Preparation method
[0049] Weigh the prescription amount of Salvia miltiorrhiza extract, microcrystalline cellulose, starch, croscarmellose sodium and carboxymethyl starch sodium and mix evenly, add water to make soft material, extrude strips, spheronize, discharge, and put the pellets in 60 Dry at ℃, sieve, and make ball cores, and take 500 grams of ball cores with the required mesh number.
[0050] Weigh the prescribed amount of ultra-fine talc powder, water, and NE-30D to make a suspension, under magnetic stirring, the ...
Embodiment 2
[0052] Release test
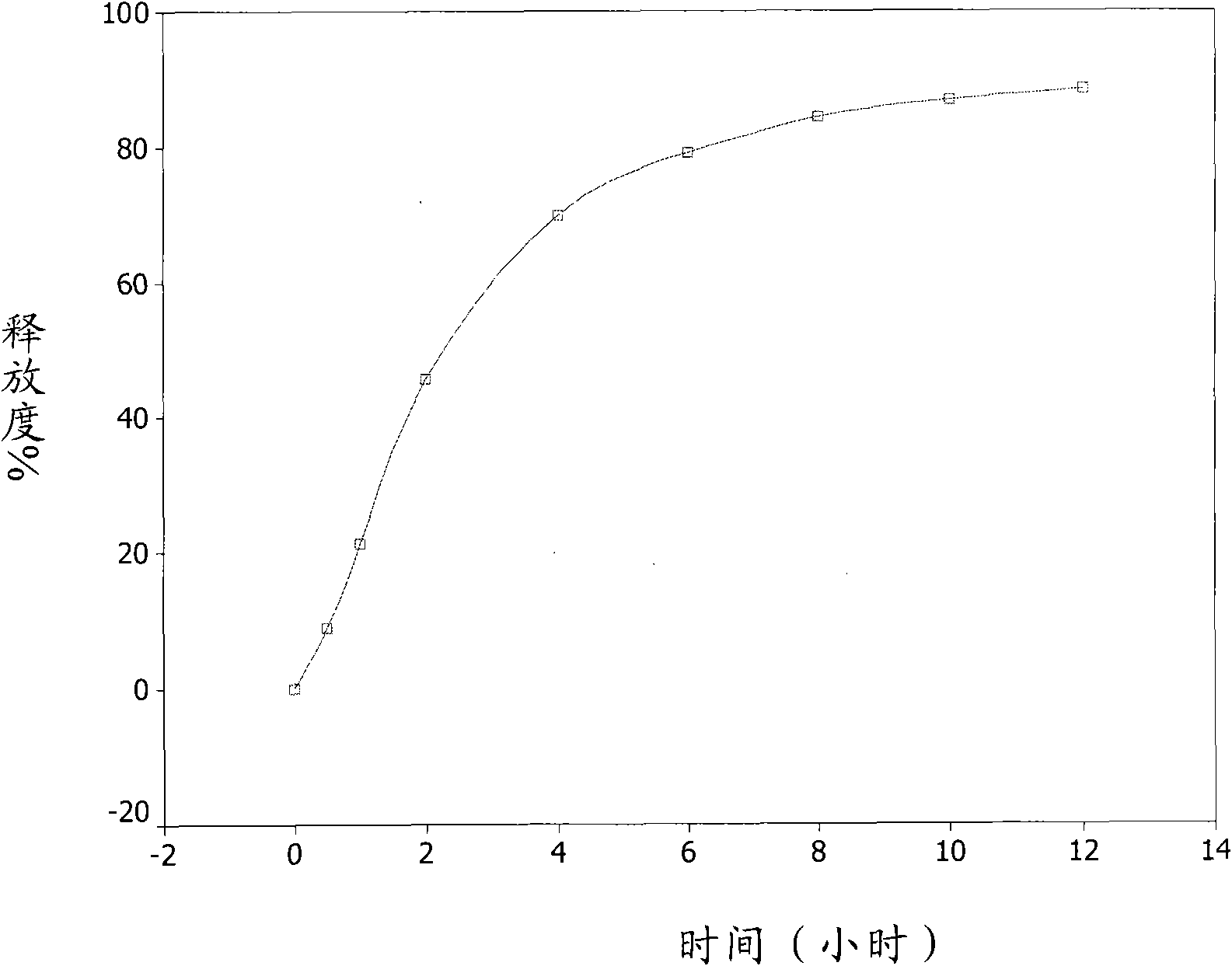
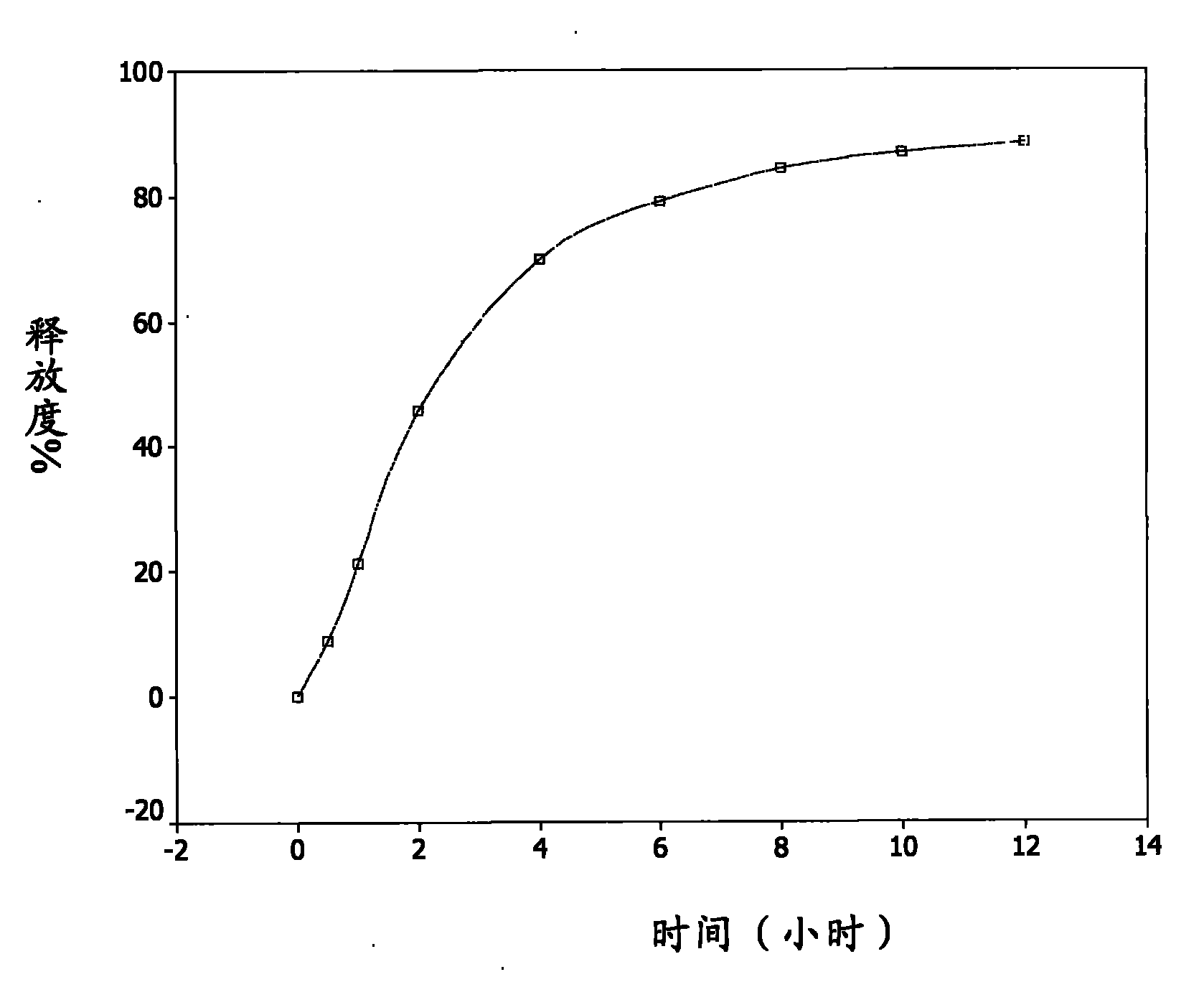
[0053] Accurately weigh about 2.0000 grams of the sustained-release pellets of Example 1, sample number 20090505, put them into a rotating basket, use 900ml of freshly boiled and cooled distilled water as the medium, and conduct a dissolution test at 37.0±0.5°C and 100 rpm. Take samples at 0.5, 1, 2, 4, 6, 8, 10, and 12 hours, filter, take 5ml of the filtrate, measure UV at 282nm, the release rate reaches 40% in the first 2 hours, and 60% in 4 hours , the release rate reached 80% in 8 hours, and finally the cumulative release percentage of the sustained-release pellets reached 88.4% in 12 hours, see attached figure 1 .
Embodiment 3
[0055] 1. Prescription
[0056] 1.1 Pill Core Prescription
[0057] Danshen Extract 150g
[0058] 450g microcrystalline cellulose
[0059] Starch 150g
[0060] Croscarmellose Sodium 20g
[0061] Sodium carboxymethyl starch 20g
[0062] 1.2 Prescription of coating solution
[0063] Ultrafine Talc Powder 1.5g
[0064] 37.5 grams of water
[0065] Copolymer of methyl methacrylate and ethyl acrylate (trade name NE-30D )
[0066] 60.0 grams
[0067] 2. Preparation method
[0068] Weigh the prescription amount of Salvia miltiorrhiza extract, microcrystalline cellulose, starch, croscarmellose sodium and carboxymethyl starch sodium and mix evenly, add water to make soft material, extrude strips, spheronize, discharge, and put the pellets in 60 Dry at ℃, sieve, and make ball cores, and take 500 grams of ball cores with the required mesh number.
[0069] Superfine talcum powder, water, and NE-30D of the prescribed amount are taken by weighing to make a suspension, and under m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com