Crystallization method of cefadroxil monohydrate and crystals
A technology of cefadroxil monohydrate and cefadroxil, which is applied in the field of cefadroxil monohydrate tetragonal flaky crystals, can solve the problems of product degradation, wide particle size distribution, and decreased yield, and achieve avoidance of agglomeration and uniform particle size distribution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
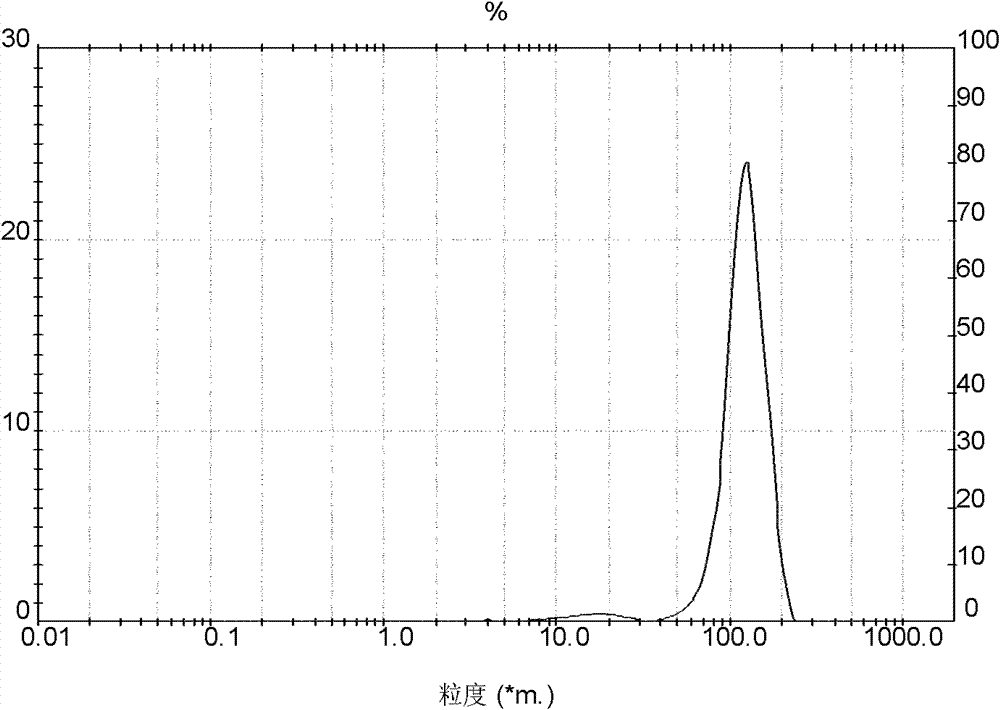
Embodiment 1
[0037] Preparation of crystalline cefadroxil monohydrate:
[0038] 1) First, 60 g of anhydrous salt water is added to the crystallizer, and then 15 g of DMF is added thereto. Add the weighed 50g cefadroxil DMF solvent compound in its mixed solvent then, maintain feed temperature simultaneously at 25 ± 1 ℃, stir to obtain the suspension of cefadroxil DMF solvent compound, and drop ammoniacal liquor at any time to keep solution The pH value is 6.7-7.0, and the concentration of ammonia water is NH 3 The content is 5% (w / w);
[0039] 2) Determine whether the cefadroxil DMF solvent compound has been completely transformed into cefadroxil monohydrate by microscope observation or refractive index detection. Treat that the crystals have all been converted, quickly turn down the stirring speed of the cefadroxil suspension, then add hydrochloric acid to adjust the pH value of the solution, and the pH value will be reduced to 4.8-5.0 through 10 minutes, and the concentration of hydroch...
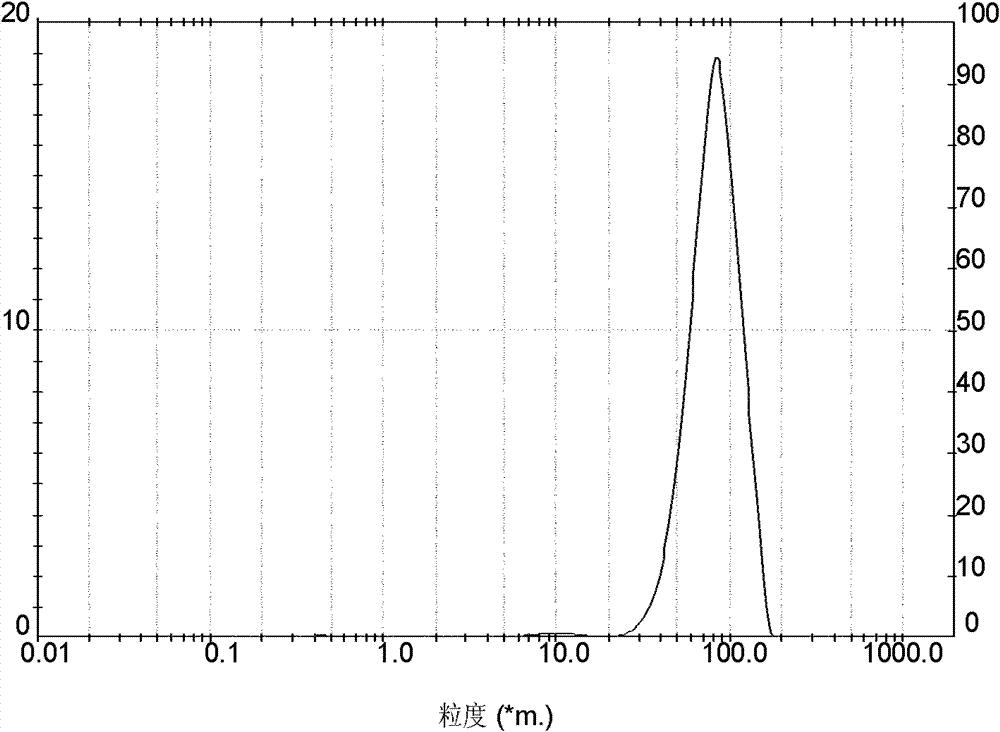
Embodiment 2
[0044] Preparation of crystalline cefadroxil monohydrate:
[0045] 1) Firstly, 200g of anhydrous salt water is added into the crystallizer, and then 20g of DMF is added thereto. Add the weighed 157g cefadroxil DMF solvent compound in its mixed solvent then, maintain feed temperature simultaneously at 25 ± 1 ℃, stir to obtain the suspension of cefadroxil DMF solvent compound, and drop ammoniacal liquor at any time to keep solution The pH value is between 6.5-6.8, and the concentration of ammonia water is NH 3 The content is 10% (w / w);
[0046] 2) Determine whether the cefadroxil DMF solvent compound has been completely transformed into cefadroxil monohydrate by microscope observation or refractive index detection. After all the crystals have been converted, slow down the stirring speed of the cefadroxil suspension, then add hydrochloric acid to adjust the pH value of the solution, and reduce the pH value to 4.8-5.0 after 15 minutes, and the concentration of hydrochloric acid ...
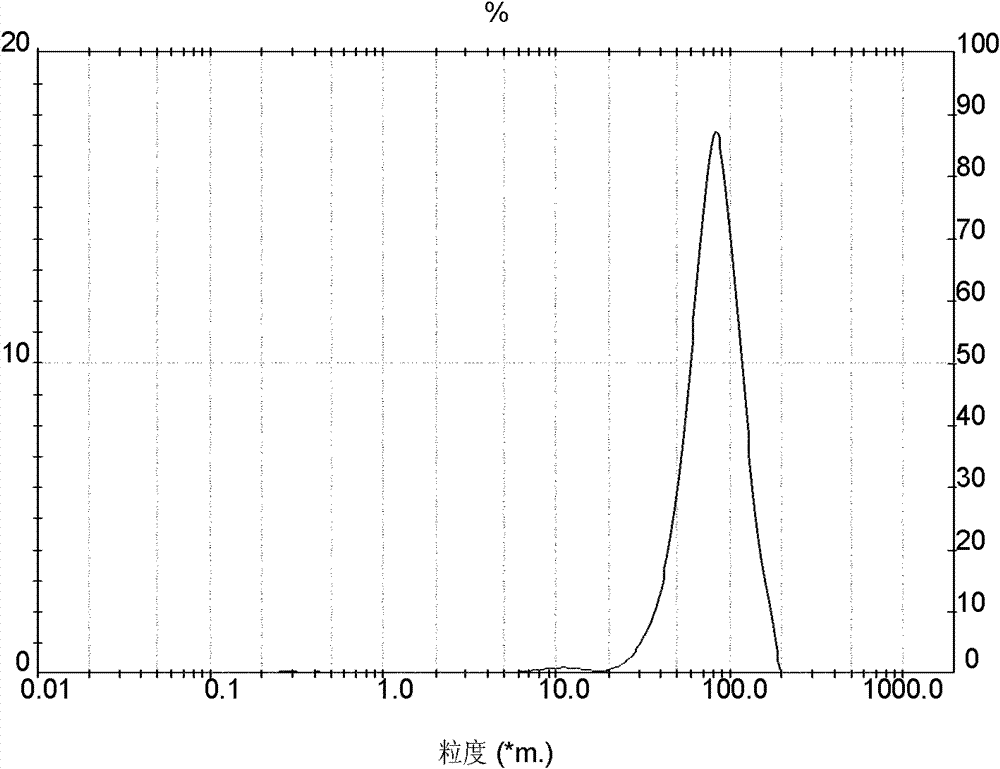
Embodiment 3
[0052] Preparation of crystalline cefadroxil monohydrate:
[0053] 1) First, 100 g of anhydrous salt water is added to the crystallizer, and then 20 g of DMF is added thereto. Add the weighed 75g cefadroxil DMF solvent compound in its mixed solvent then, maintain feed temperature simultaneously at 19 ± 1 ℃, stir to obtain the suspension of cefadroxil DMF solvent compound, and drop ammoniacal liquor at any time to keep solution The pH value is between 6.4-6.7, and the concentration of ammonia water is NH 3 The content is 10% (w / w);
[0054] 2) Determine whether the cefadroxil DMF solvent compound has been completely transformed into cefadroxil monohydrate by microscope observation or refractive index detection. After all the crystals have been converted, slow down the stirring speed of the cefadroxil suspension, then add hydrochloric acid to adjust the pH value of the solution, and reduce the pH value to 4.8-5.0 after 4 minutes, and the concentration of hydrochloric acid is 7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com