Preparation method of quantitative microorganism freeze-dried product
A technology of microbial strains and dry products, applied in the direction of biochemical equipment and methods, microorganisms, microorganisms, etc., can solve the problems of time-consuming, death of quality control strains, increase of enterprise costs, etc., and achieve the advantages of convenient use, convenient inoculation and other operations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] A method for preparing a quantitative microbial freeze-dried product, comprising the following steps:
[0028] 1) Dilute the microorganisms in the stable period to obtain a uniform dilution and make a bacterial suspension with a quantitative concentration;
[0029] 2) Mix the bacterial suspension with the protective agent to obtain a mixed solution;
[0030] 3) Bottle the quantitative mixed solution, pre-freeze below -20°C, then vacuum freeze-dry and vacuum seal to obtain quantitative microbial freeze-dried products;
[0031] Wherein, the protective agent contains 0.1-10 parts by mass of water-soluble sugar, 0.1-5 parts by mass of skimmed milk powder, 0.1-20 parts by mass of gelatin, and 0-10 parts by mass of activated carbon.
[0032] Preferably, the pre-freezing temperature is -80 to -20°C.
[0033] Preferably, the protective agent contains 3-5 parts by mass of water-soluble sugar, 0.5-3 parts by mass of skimmed milk powder, 5-13 parts by mass of gelatin, and 0.01-4...
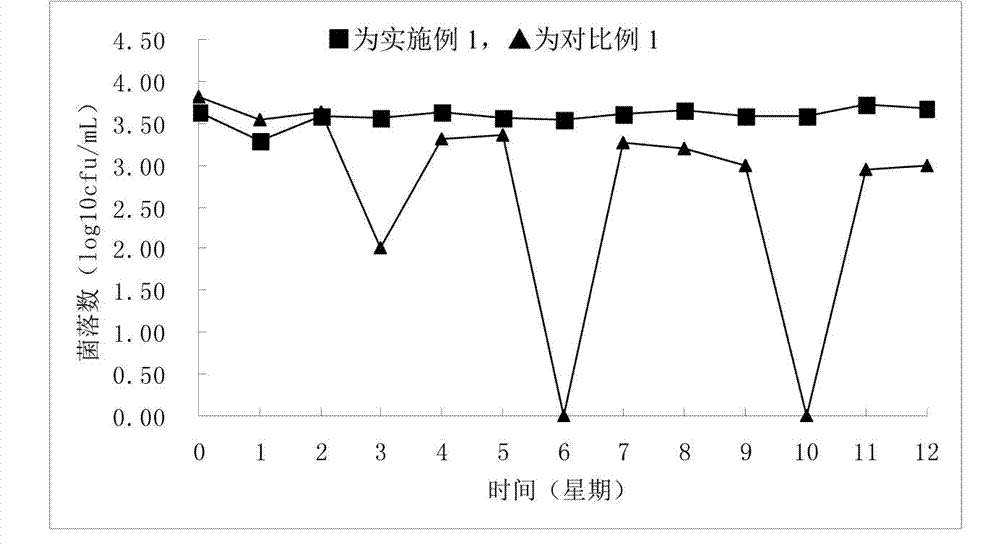
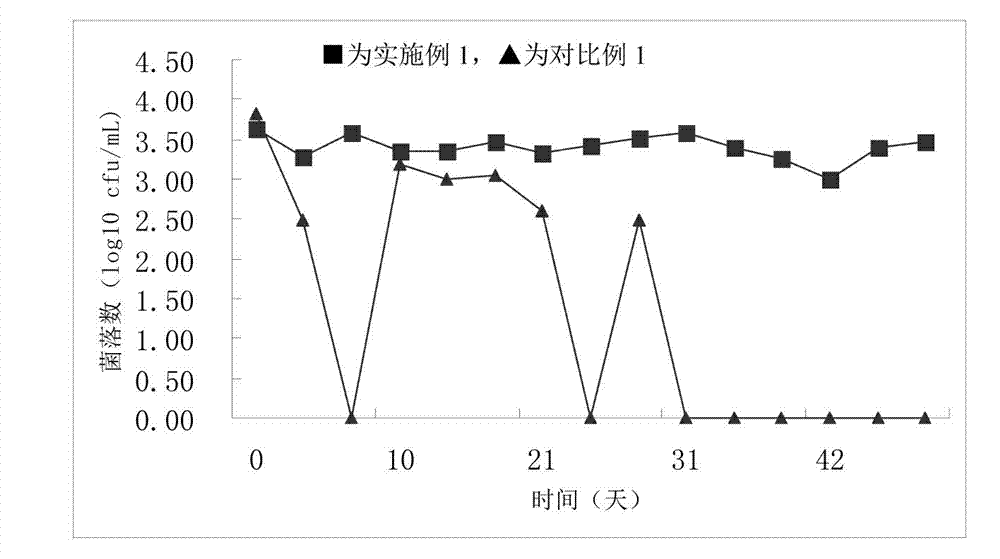
Embodiment 1
[0039] A method for preparing a quantitative microbial freeze-dried product, comprising the following steps:
[0040] 1) Dilute the standard bacterial strain Staphylococcus aureus ATCC6538 in the stable phase with 0.85% sterilized physiological saline, shake with an oscillator to obtain a uniform dilution, and make a bacterial suspension of quantitative concentration;
[0041] 2) Take quantitative bacterial suspension and protective agent by 10 8 The ratio of cfu / per gram of protective agent is mixed to obtain a mixed solution;
[0042] 3) Bottle the quantitative mixed solution, pre-freeze at -30°C, then vacuum freeze-dry, and vacuum seal to obtain quantitative microbial freeze-dried products;
[0043] Wherein, the protective agent is composed of 0.1 parts by mass of glucose, 4 parts by mass of skimmed milk powder, and 20 parts by mass of gelatin.
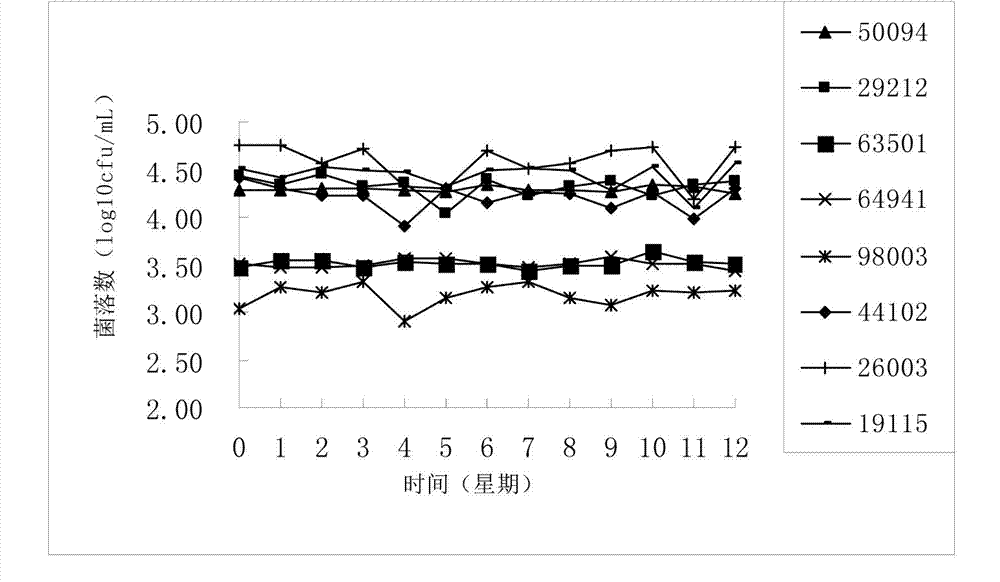
Embodiment 2
[0047] A method for preparing a quantitative microbial freeze-dried product, comprising the following steps:
[0048] 1) Dilute Escherichia coli CMCC (B) 44102 in the stable phase with 0.85% sterilized saline, shake with an oscillator to obtain a uniform dilution, and make a bacterial suspension of quantitative concentration;
[0049]2) Take quantitative bacterial suspension and protective agent by 10 6 The ratio of cfu / per gram of protective agent is mixed to obtain a mixed solution;
[0050] 3) Bottle the quantitative mixed solution, pre-freeze at -40°C, then vacuum freeze-dry, and vacuum seal to obtain quantitative microbial freeze-dried products;
[0051] Wherein, the protective agent is composed of 3 parts by mass of glucose, 3 parts by mass of skimmed milk powder, 8 parts by mass of gelatin, and 0.01 parts by mass of activated carbon.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com