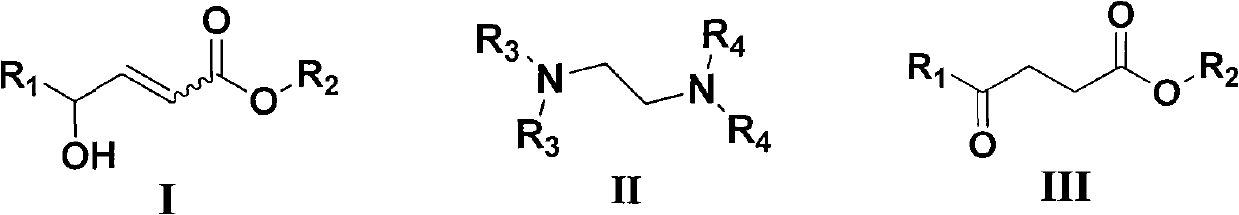
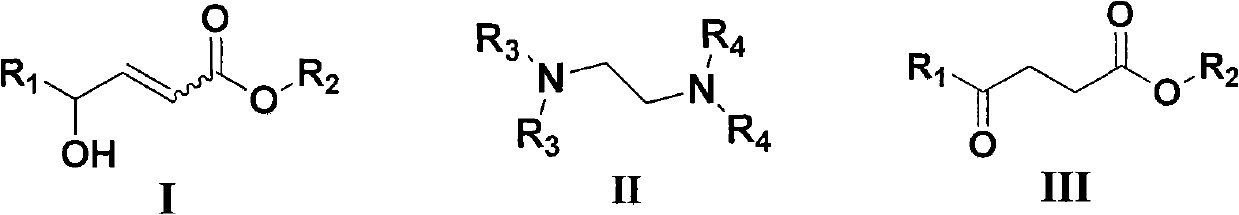
Method for synthesizing gamma-carbonyl ester compound by gamma-hydroxied gadoleic acid ester
A technology of hydroxyalkenoate and compound, applied in the field of organic intermediate synthesis, can solve the problems of respiratory system damage, carcinogenicity, difficult storage and transportation, etc., and achieves the effects of wide application, cost reduction, and avoidance of transition metal residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0026] Examples 1-10: Adding Mg(OMe) to a round bottom flask 2 (10~80mol%, 0.033M methanol solution) and catalyst IIa (IIa: R 3 =R 4 =Me, i.e. tetramethylethylenediamine, abbreviated as TMEDA, 0~100mol%), then add (Z)-γ-hydroxyalkenoate compound Ia (Ia: R 1 =C 7 h 15 , R 2 =Me) (0.2mmol), stirred at room temperature for 0.5 to 3 hours, after the thin plate chromatography detection reaction was completed, concentrated under reduced pressure to remove excess methanol, then added 5% hydrochloric acid to quench, the aqueous phase was extracted three times with ethyl acetate, combined The organic phase was washed with saturated brine, then dried over anhydrous sodium sulfate, concentrated to dryness under reduced pressure, and purified by column chromatography (petroleum ether: ethyl acetate = 12:1 ~ 9:1) to obtain a colorless liquid which was γ- Carbonyl ester compound IIIa (IIIa: R 1 =C 7 h 15 , R 2= Me). Its chemical formula represents as shown in (1):
[0027]
[...
Embodiment 11-15
[0037] Embodiment 11-15 product---gamma-carbonyl ester compound (IIIb) NMR and mass spectrum: 1 H NMR: δ7.95 (1H, s, ArH), 7.85-7.87 (1H, d, J = 7.5Hz, ArH), 7.53-7.56 (1H, d, J = 7.8Hz, ArH), 7.39-7.47 ( 1H, m, ArH), 3.71(3H, s, MeO), 3.27-3.33(q, J=6.6Hz, 2H), 2.75-2.82(q, J=6.6Hz, 2H). 13 C NMR: δ196.8, 173.1, 138.1, 135.0, 133.1, 130.0, 128.2, 126.1, 51.9, 33.5, 27.9. EI-MS (m / z): 226 (M + ), 195 (M + -31), 139(M + -87), 111 (M + -115).
Embodiment 16
[0038] Example 16: Addition of Mg(OMe) to a round bottom flask 2 (67mol%, 0.033M methanol solution) and TMEDA (50mol%), then add (E)-γ-hydroxyalkenoate compound (0.2mmol), stir at room temperature for 48 hours, after the thin plate chromatography detection reaction is completed, decompression Concentrate to remove excess methanol, then add 5% hydrochloric acid to quench, the aqueous phase is extracted three times with ethyl acetate, the combined organic phase is washed with saturated brine, then dried with anhydrous sodium sulfate, concentrated to dryness under reduced pressure, and purified by column chromatography (Petroleum ether: ethyl acetate = 12:1-9:1) to obtain a colorless liquid which is the γ-carbonyl ester compound (the product is the same as in Example 17).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com