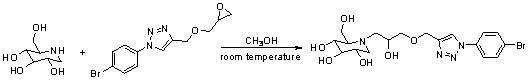
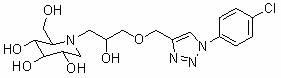
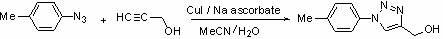Use of derivatives of 1-deoxynojiri mycin containing 1,2,3-triazoles as alpha-glucosidase inhibitors
A technology of deoxynojirimycin and glucosidase, applied in antiviral agents, drug combinations, metabolic diseases, etc., can solve the problems of differences in inhibitor enzyme levels and cell levels, difficulty in artificial synthesis, low DNJ content, etc., to achieve Easy operation, mild reaction conditions, and the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Synthesis of hydroxymethyl-containing aromatic triazole derivative 2a
[0045]
[0046] 2a
[0047] Add p-methylazidobenzene (119 mg, 1 mmol), propynyl alcohol (96 μL, 1.2 mmol), sodium ascorbate (20 mg, 0.1 mmol), cuprous iodide (19 mg, 0.1 mmol) to the reaction In the bottle, under the protection of nitrogen, react at room temperature for 24 hours. After TLC detection shows that the reaction is complete, add water to the reaction system, extract with ethyl acetate, wash the organic phase with water, dry over anhydrous sodium sulfate, and remove ethyl acetate by vacuum rotary evaporation. Silica gel After column separation, 114 mg of a light yellow solid was obtained, with a yield of 60%.
[0048] (2) Synthesis of triazole-containing epoxy compound 3a
[0049]
[0050] 3a
[0051] Add epichlorohydrin (390 μL, 5 mmol), TBAB (16 mg) and 50% aqueous sodium hydroxide solution (0.6 mL) into the reaction flask, stir vigorously at room temperature until completely ...
Embodiment 2
[0058] (1) Synthesis of hydroxymethyl-containing aromatic triazole derivative 2b
[0059]
[0060] 2b
[0061] Add p-fluoroazide (137 mg, 1 mmol), propynyl alcohol (96 μL, 1.2 mmol), sodium ascorbate (20 mg, 0.1 mmol), cuprous iodide (19 mg, 0.1 mmol) into the reaction flask , under the protection of nitrogen, react at room temperature for 24 hours, after TLC detection shows that the reaction is complete, add water to the reaction system, extract with ethyl acetate, wash the organic phase with water, dry over anhydrous sodium sulfate, remove ethyl acetate by vacuum rotary evaporation, silica gel column After separation, 172 mg of yellow solid was obtained with a yield of 89%.
[0062] (2) Synthesis of triazole-containing epoxy compound 3b
[0063]
[0064] 3b
[0065] Add epichlorohydrin (390 μL, 5 mmol), TBAB (16 mg) and 50% aqueous sodium hydroxide solution (0.6 mL) into the reaction flask, stir vigorously at room temperature until completely dissolved, then place i...
Embodiment 3
[0072] (1) Synthesis of hydroxymethyl-containing aromatic triazole derivative 2c
[0073]
[0074] 2c
[0075] Add p-chloroazidobenzene (154 mg, 1 mmol), propynyl alcohol (96 μL, 1.2 mmol), sodium ascorbate (20 mg, 0.1 mmol), cuprous iodide (19 mg, 0.1 mmol) into the reaction flask , under the protection of nitrogen, react at room temperature for 24 hours, after TLC detection shows that the reaction is complete, add water to the reaction system, extract with ethyl acetate, wash the organic phase with water, dry over anhydrous sodium sulfate, remove ethyl acetate by vacuum rotary evaporation, silica gel column After separation, 189 mg of reddish-brown solid was obtained, with a yield of 90%.
[0076] (2) Synthesis of triazole-containing epoxy compound 3c
[0077]
[0078] 3c
[0079] Add epichlorohydrin (390 μL, 5 mmol), TBAB (16 mg) and 50% aqueous sodium hydroxide solution (0.6 mL) into the reaction flask, stir vigorously at room temperature until completely dissolve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com