Poly(gamma-propynyl-L-glutamate) block copolymer and preparation method and hydrogel thereof
A technology of block copolymers and glutamate, which is applied in drug delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as unfavorable hydrogel applications, and achieve long-lasting bioactive molecule release capabilities, Good biocompatibility and the effect of expanding the application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The present invention also provides a kind of preparation method of poly(γ-propynyl-L-glutamate) block copolymer, comprises the following steps:
[0045] Aminated polyethylene glycol or aminated polyethylene glycol monomethyl ether and γ-propynyl-L-glutamic acid ester-N-carboxylic acid anhydride are polymerized in an organic solvent to obtain poly(γ - propynyl-L-glutamate) block copolymer;
[0046] The aminated polyethylene glycol monomethyl ether has the structure of formula (IV), and the number average molecular weight is 550-10000:
[0047]
[0048] The aminated polyethylene glycol has a structure of formula (V), and the number average molecular weight is 550-10000:
[0049]
[0050] The molar ratio of the aminated polyethylene glycol or aminated polyethylene glycol monomethyl ether to γ-propynyl-L-glutamic acid ester-N-carboxylic acid anhydride is 1:5-120.
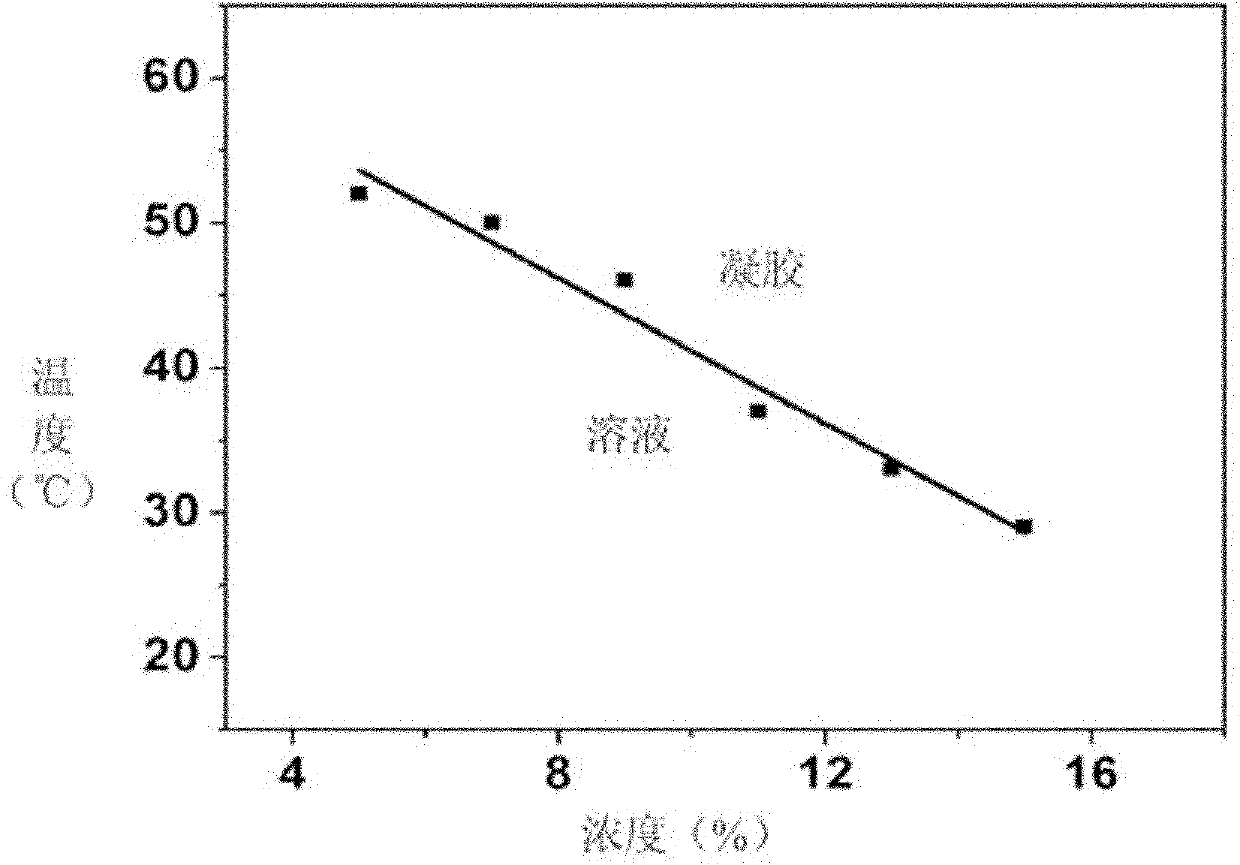
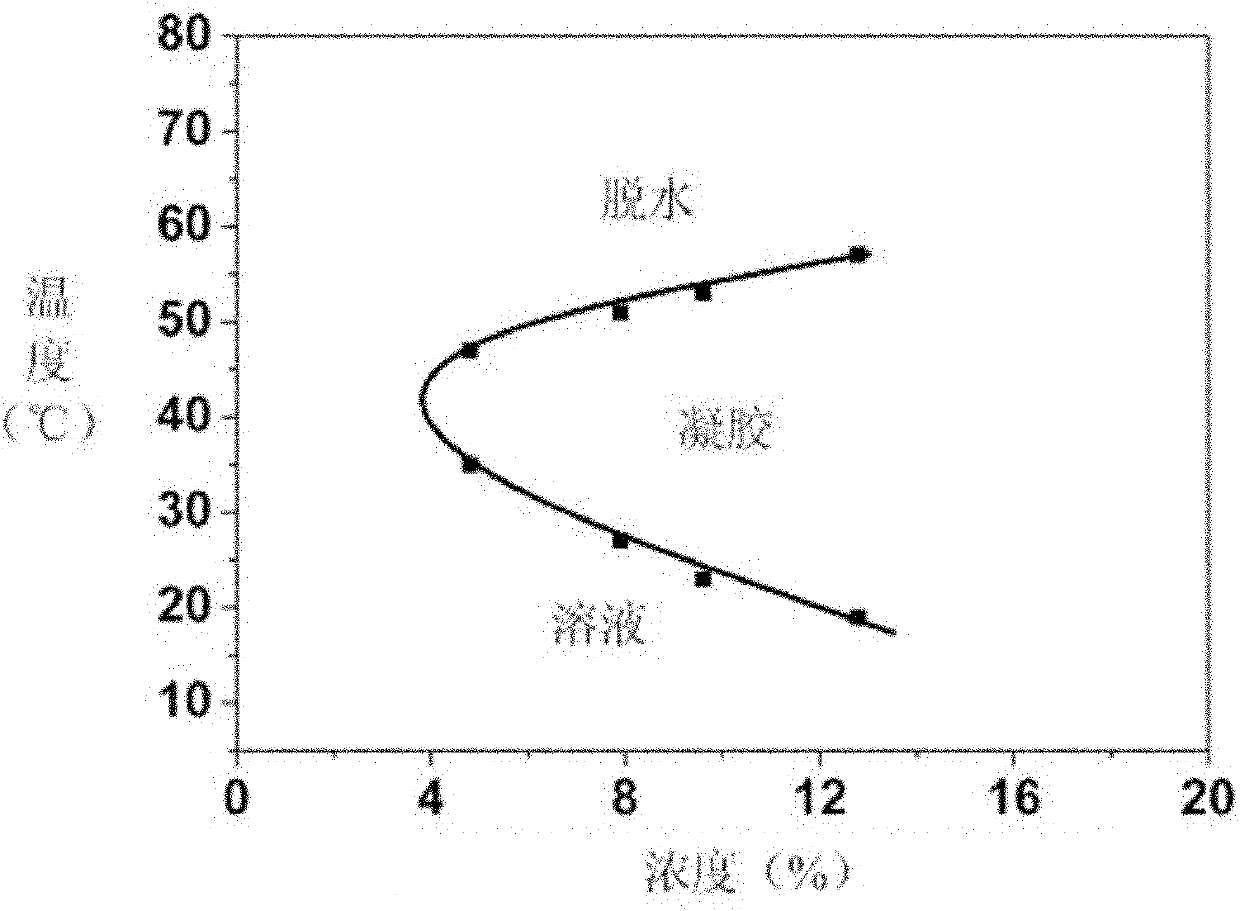
[0051] Aminated polyethylene glycol monomethyl ether and γ-propynyl-L-glutamic acid ester-N-carboxyli...
Embodiment 1
[0076]Add 2.2 g of aminated polyethylene glycol monomethyl ether with a number-average molecular weight of 550 to the dry reaction flask, remove water with 60 mL of anhydrous toluene at 130 ° C for 2 h, and vacuum dry the remaining Toluene; the obtained solid was dissolved in 20 mL of dry N,N-dimethylformamide to obtain the first solution; 4.22 g of γ-propynyl-L-glutamate-N-carboxylic acid anhydride was dissolved in 40 mL of dry N, N-dimethylformamide, to obtain the second solution; in a nitrogen atmosphere, the first solution and the second solution were mixed, stirred and reacted at room temperature and under nitrogen protection conditions for 24h; after the reaction, pumped under reduced pressure Dry N, N-dimethylformamide, then dissolve the obtained solid in chloroform, then settle with ether, filter with suction, and dry to obtain polyethylene glycol monomethyl ether-poly(γ-propynyl- L-glutamate) block copolymer.
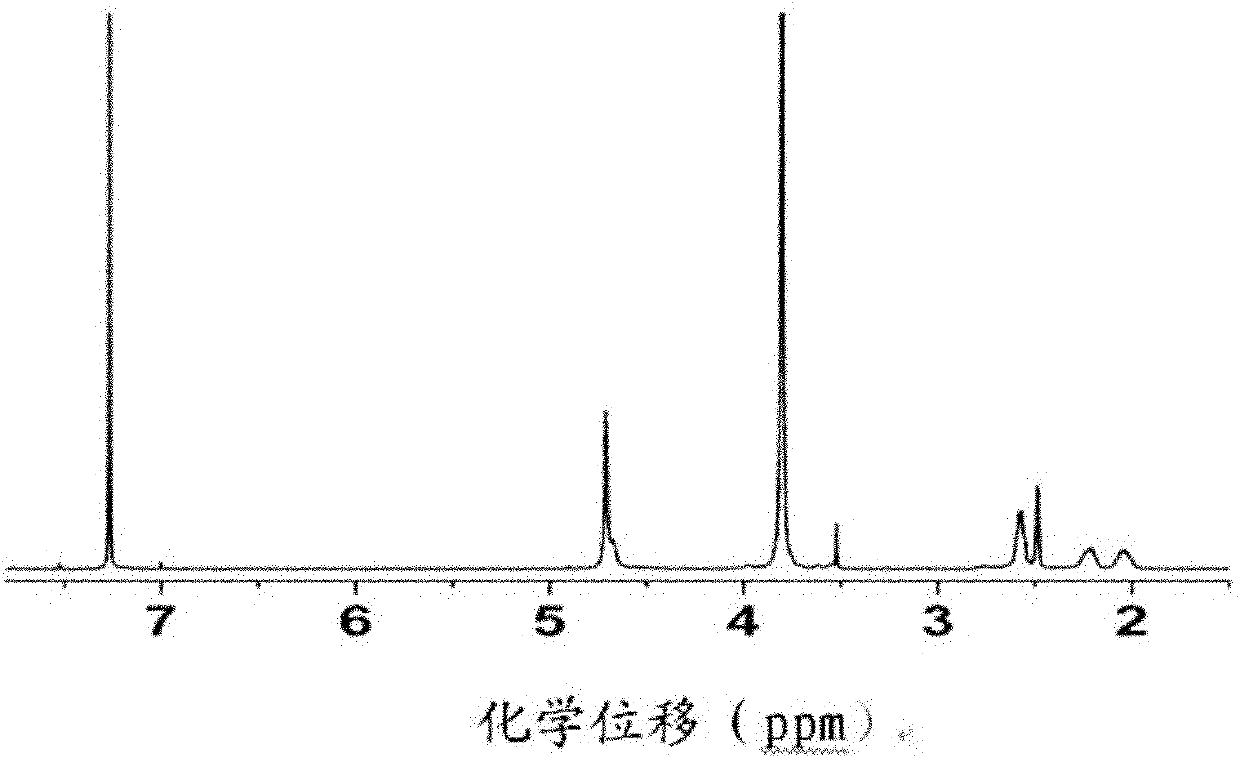
[0077] Carry out nuclear magnetic resonance analysis to ...
Embodiment 2
[0079] Add 2.2 g of amino-terminated polyethylene glycol with a number average molecular weight of 550 to the dry reaction bottle, and remove the water with 60 mL of anhydrous toluene at 130 ° C for 2 hours, then vacuum the remaining toluene to dry up; The obtained solid was dissolved in 20 mL of dry N,N-dimethylformamide to obtain the first solution; 6.7 g of γ-propynyl-L-glutamate-N-carboxylic acid anhydride was dissolved in 50 mL of dry N, In N-dimethylformamide, the second solution was obtained; in a nitrogen atmosphere, the first solution was mixed with the second solution, and stirred and reacted for 48h at room temperature under nitrogen protection conditions; after the reaction was completed, the N was drained under reduced pressure, N-dimethylformamide, then dissolve the resulting solid in chloroform, settle with ether, filter with suction, and dry to obtain poly(γ-propynyl-L-glutamate)-polyethylene diethyl ether Alcohol-poly(γ-propynyl-L-glutamate) block copolymer. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com