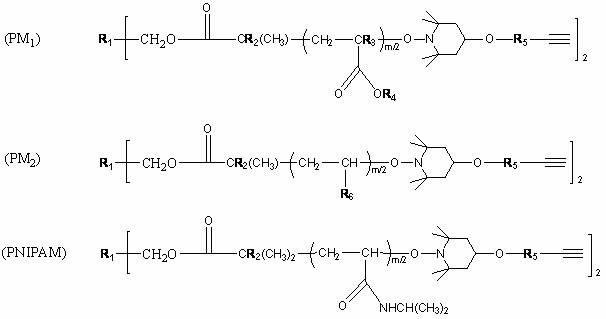
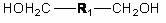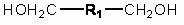Circular polymer and preparation method thereof
A technology of polymers and polymer chains, applied in the field of cyclic polymers and their preparation, can solve the problems of restricting the research and application exploration of cyclic polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of circular PS
[0053] 1. Preparation of PS with hydroxyl groups at both ends
[0054] A 500 mL ampoule was vacuum-degassed for 2 h under infrared lamp baking, and after purging oxygen with nitrogen, 4 mL of dry tetrahydrofuran, 150 mL of cyclohexane and 15.0 mL of styrene were added to the ampoule. The calculated amount of naphthalenelithium initiator (5.0 mL, 4.00 mmol) was quickly injected with a syringe. The reaction was continued at room temperature for 3 h, then EO (3.0 mL, 59 mmol) was added by syringe, and methanol was added after 12 h to terminate the reaction. The resulting solution was concentrated, precipitated in methanol, and dried under vacuum at 45 °C for 12 h to obtain a white polymer powder, namely PS with hydroxyl groups at both ends, M n,SEC =3400g / mol, PDI=1.05. Its reaction formula is:
[0055] .
[0056] . Preparation of PS with propargyl at both ends
[0057] Add about 8.0 g of the above PS with hydroxyl groups at...
Embodiment 2
[0062] Example 2 Preparation of circular PB
[0063] The styrene in Example 1 was replaced with butadiene, namely to synthesize a ring-shaped PB polymer.
Embodiment 3
[0064] Example 3 Preparation of Ring PI
[0065] The styrene in Example 1 was replaced with isoprene, that is, a ring-shaped PI polymer was synthesized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com