Method for producing 2,4-dichlorphenoxyacetic acid
A technology of dichlorophenoxyacetic acid and phenoxyacetic acid, applied in chemical instruments and methods, preparation of carboxylate salts, preparation of organic compounds, etc., can solve problems such as post-processing difficulties, environmental pollution, and small amount of waste disposal, and achieve Small amount of waste treatment, reduced production cost, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
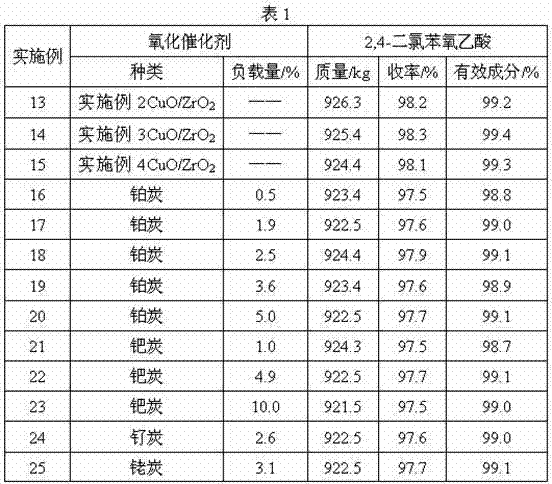
[0026] Preparation of CuO / ZrO 2 Oxidation catalyst: with Na 2 CO 3 solution (5mol / L) to precipitate ZrOCl 2 ·8H 2 O(2.5mol / L) and Cu(NO 3 ) 2 ·3H 2 A mixed solution of O (2.5mol / L) (the molar ratio of zirconium ions and copper ions in the mixed solution is 1:1.5), the precipitation temperature is 65°C, and the final pH is 7.9. After the obtained hydrogel was stirred for 35 minutes, it was washed with deionized water to remove chloride ions and sodium ions, dried at 108°C, and calcined at 350°C to obtain CuO / ZrO 2 catalyst.
Embodiment 2
[0028] Preparation of CuO / ZrO 2 Oxidation catalyst: with Na 2 CO 3 solution (3mol / L) to precipitate ZrOCl 2 ·8H 2 O and Cu(NO 3 ) 2 ·3H 2 A mixed solution of O (the molar ratio of zirconium ions and copper ions in the mixed solution is 1:1.0), the precipitation temperature is 60° C., and the final pH is 8.2. After the obtained hydrogel was stirred for 45 minutes, it was washed with deionized water to remove chloride ions and sodium ions, etc., dried at 105°C, and calcined at 300°C to obtain CuO / ZrO 2 catalyst.
Embodiment 3
[0030] Preparation of CuO / ZrO 2 Oxidation catalyst: with Na 2 CO 3 solution (6mol / L) to precipitate ZrOCl 2 ·8H 2 O and Cu(NO 3 ) 2 ·3H 2The mixed solution of O (the molar ratio of zirconium ions and copper ions in the mixed solution is 1:1.9), the precipitation temperature is 70°C, and the final pH is 7.5. After the obtained hydrogel was stirred for 30 minutes, it was washed with deionized water to remove chloride ions and sodium ions, dried at 115°C, and calcined at 450°C to obtain CuO / ZrO 2 catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com