Metoprolol Tartaric Acid and Felodipine slow-release double-layer tablet and preparation method thereof
A technology for a sustained-release layer of metoprolol tartrate and torolol, which is applied to sustained-release double-layer tablets and preparation thereof, and the sustained-release double-layer tablet comprising metoprolol tartrate and felodipine and the field of preparation thereof. Solve the problems of damaged coating film, difficult operation, long process, etc., to achieve the effect of being beneficial to industrial production, reliable slow release effect and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 sustained-release double-layer tablet prescription (1000 tablets amount)
[0045] Metoprolol tartrate layer:
[0046] Metoprolol Tartrate 50g
[0047] Ethylcellulose powder 150g
[0048] 30% (g / L) ethyl cellulose aqueous suspension 80g
[0049] Hydroxypropyl methylcellulose (viscosity 100000cp) 130g
[0050] Hydroxypropyl methylcellulose (viscosity 50cp) 50g
[0051] Magnesium Stearate 4.6g
[0052] Felodipine layer:
[0053] Felodipine 5g
[0054] Povidone k29 / 32 45g
[0055] Hydroxypropyl methylcellulose (viscosity 15000cp) 30g
[0056] Hydroxypropyl methylcellulose (viscosity 50cp) 30g
[0057] Lactose 190g
[0059] Coating solution:
[0060] Hydroxypropyl methylcellulose (viscosity 50cp) 20g
[0061] Titanium dioxide 16g
[0063] Polysorbate 80 8g
[0064] Ethanol 400g
[0065] 500ml of water.
Embodiment 2
[0066] Embodiment 2 Preparation of Metoprolol Felodipine Tartrate Sustained-release Double-Layer Tablet
[0067] According to the distribution ratio of each component shown in the prescription of Example 1, respectively prepare metoprolol tartrate sustained-release layer granules and felodipine sustained-release layer granules, and then use the mold to compress them into double-layer tablets, and take the double-layer tablets Place the formulation in a coating pan, blow hot air to 30-40°C, adjust the spray speed to coat, increase the weight of the double-layer tablet by 2%, and dry and solidify the coated tablet to obtain the finished product.
[0068] Concrete preparation process is as follows:
[0069] The preparation technology of metoprolol tartrate sustained-release layer granule comprises the following steps:
[0070] (1) Metoprolol tartrate is crossed 80 mesh sieves, for subsequent use;
[0071] (2) Metoprolol tartrate and ethyl cellulose powder of prescription quanti...
Embodiment 3
[0080] The selection of embodiment 3 metoprolol tartrate sustained-release layer formula
[0081] Considering the high solubility of metoprolol tartrate in water, first granulate metoprolol tartrate with water-insoluble material-ethyl cellulose, and then use high-viscosity hydroxypropyl methylcellulose to prepare slow release layer.
[0082] Make 5 grams of metoprolol tartrate and ethyl cellulose through a 80-100 mesh sieve to make a soft material, granulate, dry, and granulate, and then mix the obtained granules with hydroxypropyl methylcellulose evenly, and then add Magnesium stearate.
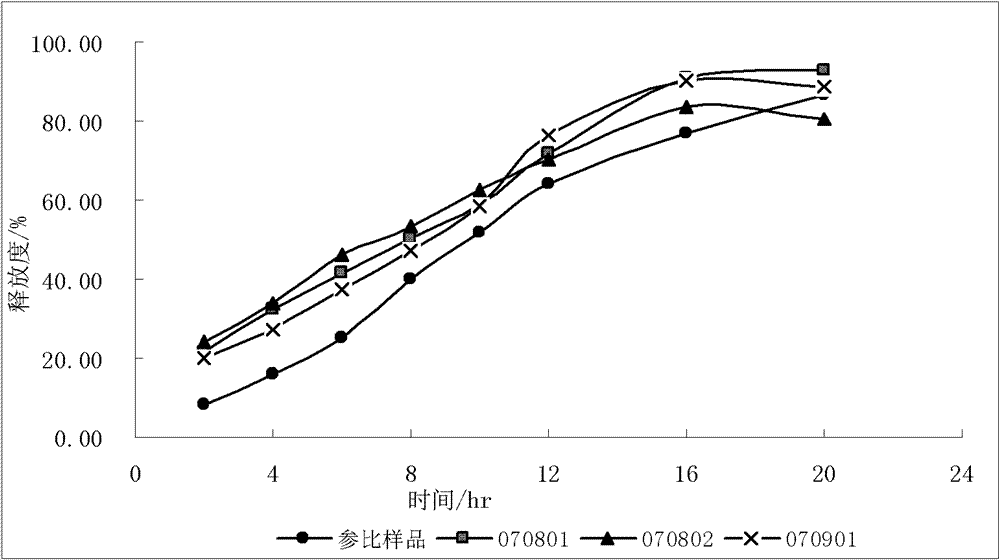
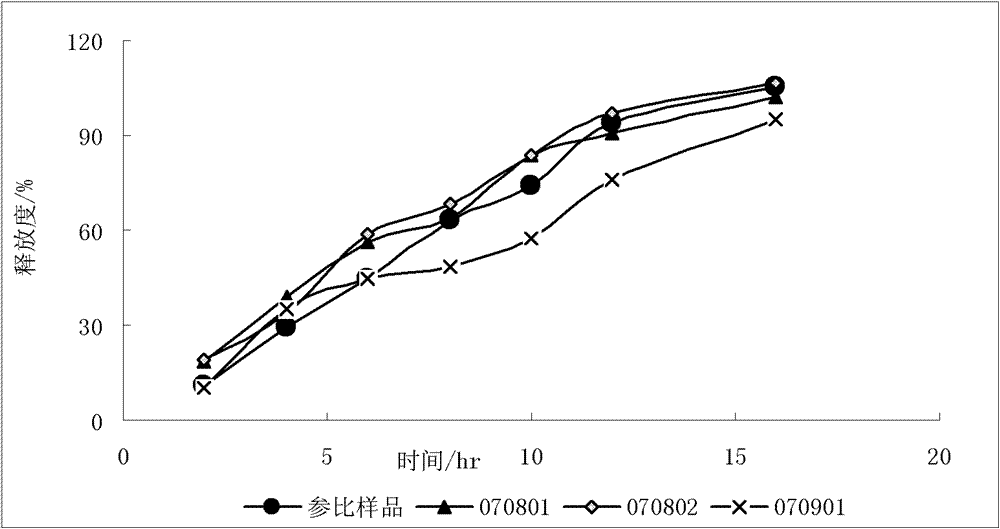
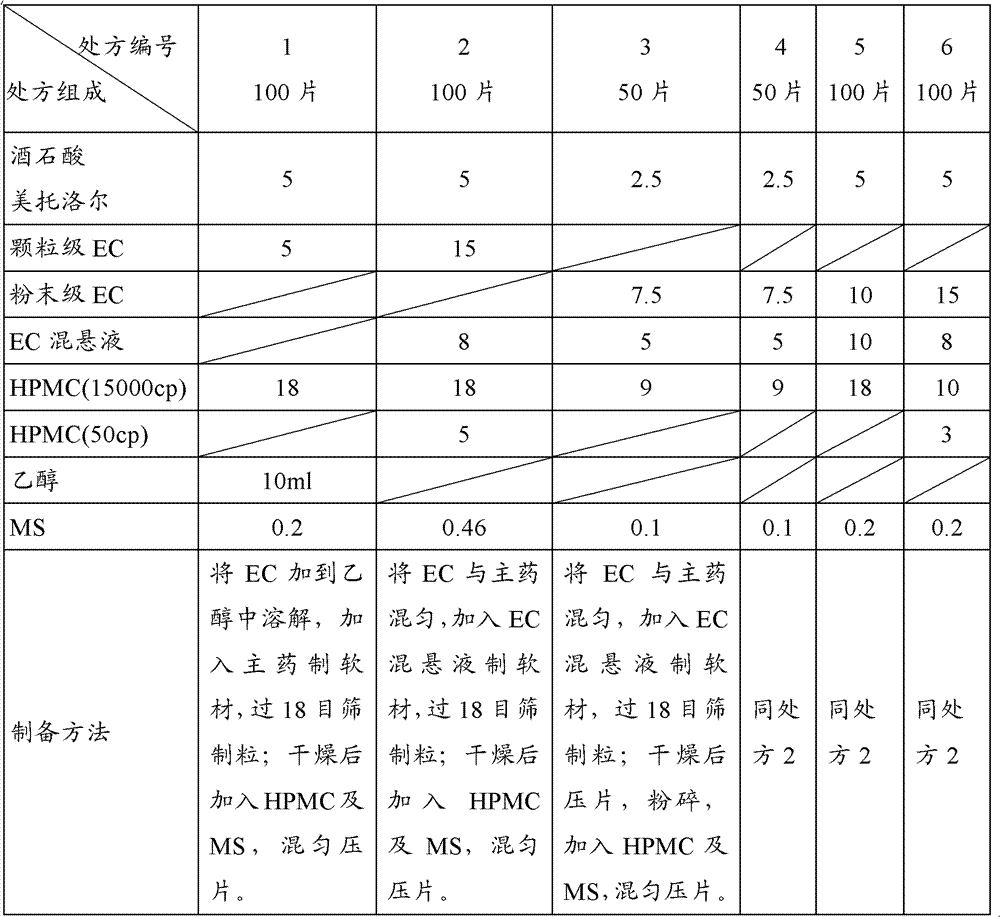
[0083] The influence of factors such as the addition forms of different ethyl cellulose, different viscosities of hydroxypropyl methylcellulose, and the amount of hydroxypropyl methylcellulose were investigated. The screening results are as follows:
[0084] Table 1 Screening of the prescription of metoprolol tartrate sustained-release layer
[0085]
[0086] Note: EC-ethyl cellulose; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com