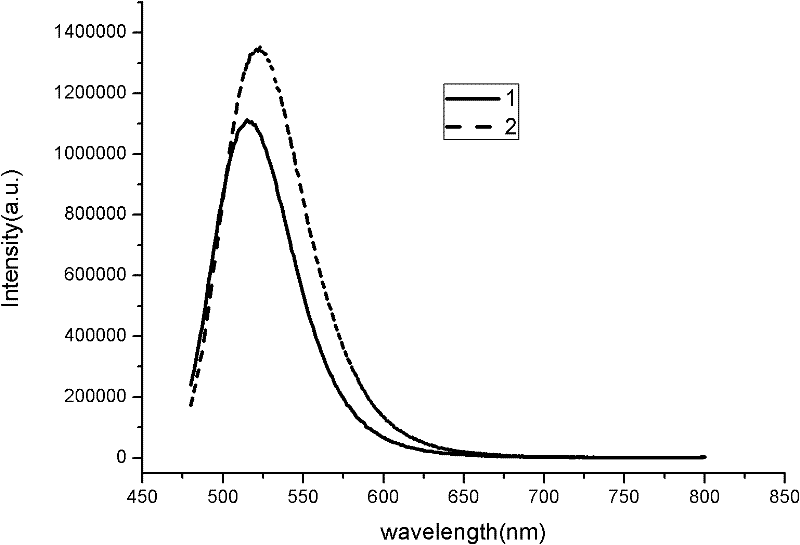
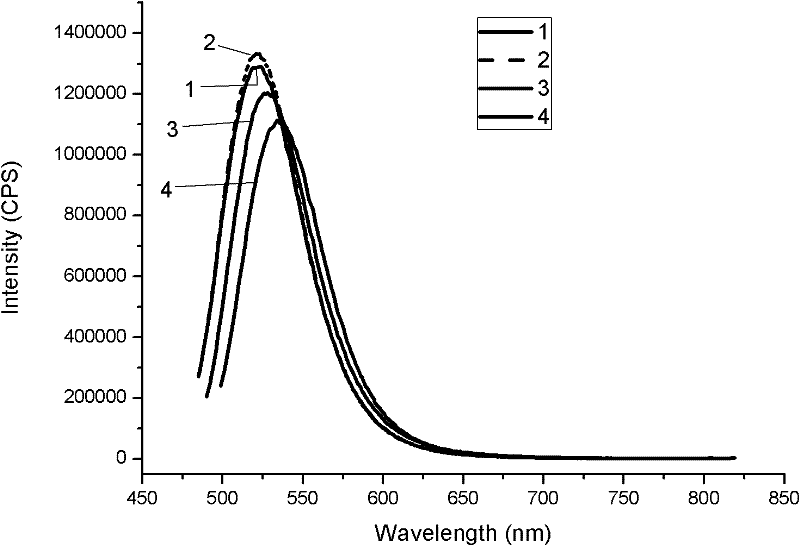
Method for optimizing crystalline form of silicate green fluorescent powder material
A technology of green phosphor and silicate, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of unstable crystal shape, low sintering temperature of phosphor powder, and poor crystallization effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The first step is to synthesize the precursor of silicate green phosphor Sr 2 SiO 4 :
[0028] Weigh SrCO according to the molar ratio Sr:Si=2:1 3 147.61g, SiO 2 30.04, and grind it, mix it, and then add flux NH of 3wt% by weight of the mixture 4 Cl, put in an alumina crucible, place the crucible in a tube furnace, sinter at 1300°C for 3 hours in a protective gas, and then cool to obtain Sr 2 SiO 4 , the protective gas is nitrogen, and the gas flow rate is 15ml / min.
[0029] In the second step, in the prebody Sr 2 SiO 4 The provided matrix structure is doped with Ba element to synthesize the silicate green phosphor material BaSr 0.98 SiO 4 :0.02Eu:
[0030] According to BaSr 0.98 SiO 4 : The molar ratio of 0.02Eu element (Sr, Ba):Si:Eu=(1-0.02):1:0.02 Molar ratio weighs BaCO 3 5g, SiO 2 0.776g and Eu 2 o 3 0.089g, then the resulting Sr 2 SiO 4 After grinding and sieving, weigh 3.318g, and after grinding and mixing, add 5wt% of the total amount of ...
Embodiment 2
[0034] In order to show the advantages of the optimized silicate green phosphor material crystal form of the present invention, so the first two steps of embodiment 2 are consistent with embodiment 1, the difference is:
[0035] In step 1), Sr element may be Ca element, and Ca element is introduced into the system through its carbonate, nitrate or oxide. Add 2wt% or 4wt% co-solvent NH in the primary mixture to the total amount of the primary mixture 4 Cl; in protective gas Ar, N 2 / H 2 , NH 3 Sintering under one or a combination of several conditions, the gas flow rate is 20ml / min, the sintering temperature is 1000°C, and the sintering time is 4 hours.
[0036] In step 2), the Sr element may be Ca element, and the Ca element is introduced into the system through its carbonate, nitrate or oxide. Add the auxiliary solvent BaF that accounts for 3wt% or 4wt% of secondary mixture total amount in secondary mixture 2 ; in protective gas H 2 , N 2 / H 2 , NH 3 Sintering under ...
Embodiment 3
[0040] The first two steps of embodiment 3 are consistent with embodiment 1, and the parameters of the third step are changed emphatically to contrast with each embodiment.
[0041] The third step is to optimize the silicate green phosphor material BaSr 0.98 SiO 4 : The crystal morphology of 0.02Eu: the BaSr obtained in the second step 0.98 SiO 4 : 0.02Eu, after cooling, take out and grind, wash, dry, weigh and this sintered product BaSr 0.98 SiO 4 : 0.02Eu weight of 8% nitride Si 3 N 4 mixed grinding, where BaSr 0.98 SiO 4 :0.02Eu is 8.5g, Si 3 N 4 It is 0.425g, put into molybdenum crucible after above-mentioned each composition is ground, crucible is moved in the high-temperature tubular furnace, in N 2 :H 2 = Sintering at 1550°C for 10 hours in a 3:1 atmosphere, with a gas flow rate of 15ml / min, then cooling to room temperature, taking out, grinding and sieving, washing and drying with ethanol to obtain the silicate green phosphor material BaSr with optimized cry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com