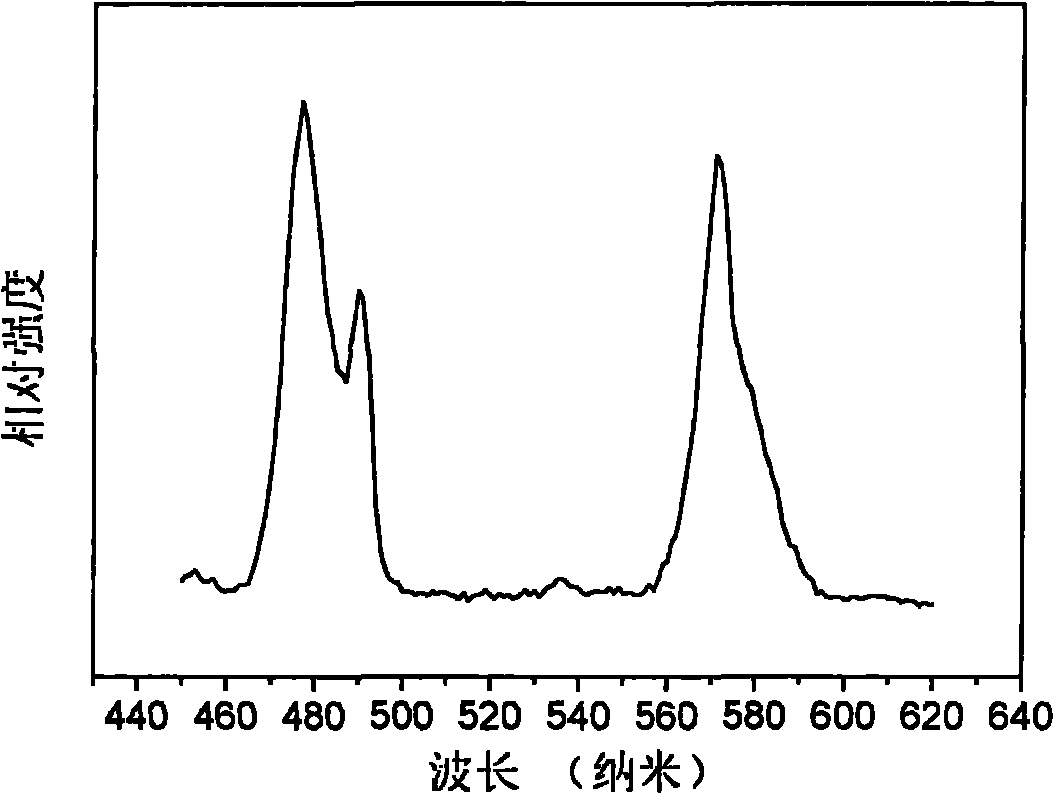
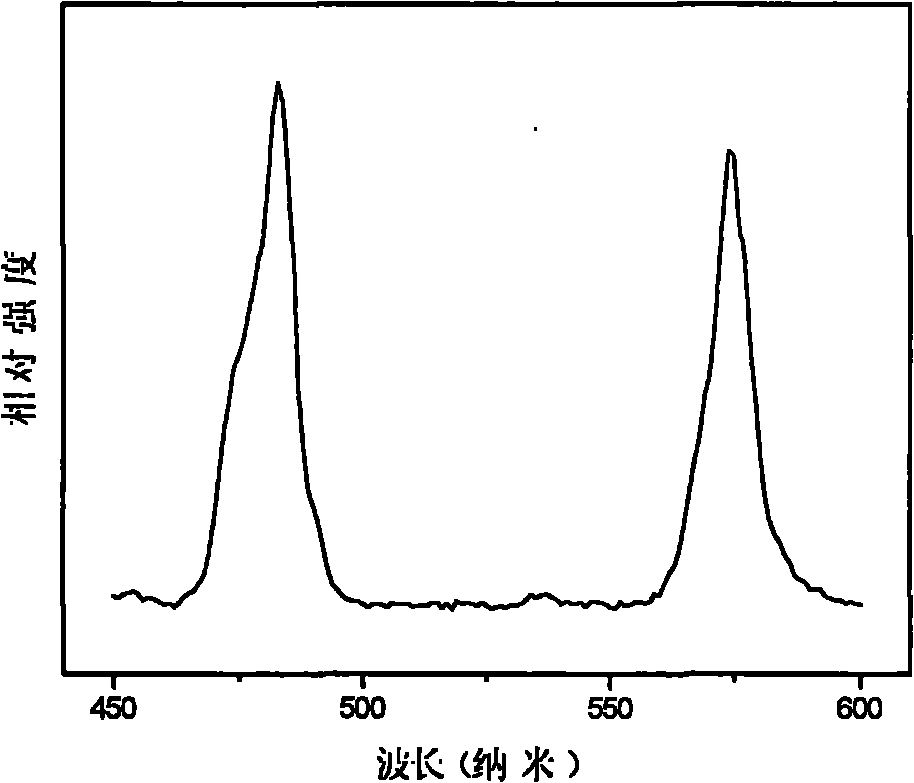
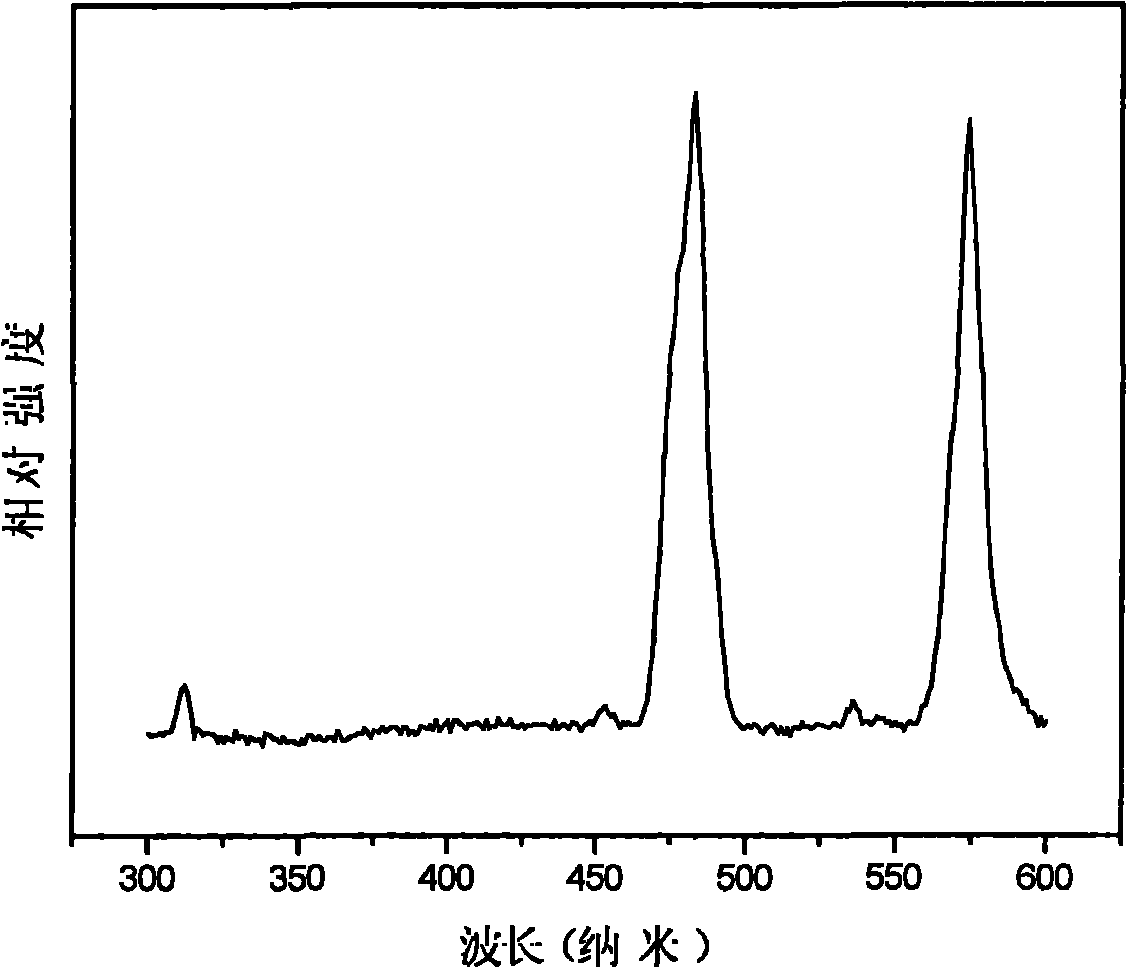
Fluophosphate-based light-emitting material and preparation method thereof
A technology of luminescent materials and fluorophosphates, applied in luminescent materials, chemical instruments and methods, etc., can solve problems such as insufficient luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] see Figure 14 , illustrating the flow of the preparation method of the fluorophosphate-based luminescent material according to the embodiment of the present invention, the preparation method includes the following steps:
[0054] S01: Select the raw material, that is, according to the chemical formula M y Re 1-x ln x PO 4 f y The molar ratio of the corresponding elements in the selected alkali metal ion source compound, the source compound of the phosphate ion, the source compound of the rare earth ion, the source compound of the Ln ion and the source compound of the fluoride ion, the source compound of the phosphate ion is molar Specific excess 5%-30%, M is an alkali metal element, Re is a rare earth element, Ln is Dy or Eu, x=0.005-0.5, y=1 or 2;
[0055] S02: mixing each source compound;
[0056] S03: pre-sintering the mixture, and then cooling;
[0057] S04: taking out the pre-sintered product, grinding it, and then calcining the ground product to obtain the...
Embodiment 1
[0064] Weigh (NH 4 ) 2 HPO 4 0.7263 g, Na 2 CO 3 0.5299 grams, Gd 2 o 3 0.9017g, Dy 2 o 3 0.0047 g, NH 4 F 0.5185 (excess 40%, mole fraction, the same below) After the raw materials are ground and mixed thoroughly, they are first sintered in an air atmosphere at 300°C for 3 hours, taken out and ground evenly, then calcined in an air atmosphere at 800°C for 8 hours, and cooled naturally to At room temperature, take it out and grind it finely to get Na 2 Gd 0.995 Dy 0.005 PO 4 f 2 white phosphor. Of which (NH 4 ) 2 HPO 4 Relative to this chemical formula, the molar ratio is excessive by 10%, that is, according to the chemical formula, the molar ratio of Na and P is 2: 1, and the molar ratio of Na and P in the raw materials provided in this embodiment is 2: 1.1, NH 4 F is 40% in excess relative to the molar ratio in the chemical formula, and the calculation method is the same as above, and the excess calculation method of each raw material in the following exa...
Embodiment 2
[0066] Weigh (NH 4 ) 2 HPO 4 0.7263 g, Na 2 CO 3 0.4240 g, K 2 CO 3 0.1382 grams, Gd 2 o 3 0.8881g, Dy 2 o 3 0.0187 g, NH 4 F 0.5185 grams of raw materials are ground and mixed thoroughly, then sintered in an air atmosphere at 300°C for 3 hours, taken out and ground evenly, then calcined in an air atmosphere at 650°C for 6 hours, cooled naturally to room temperature, taken out and ground to obtain Na 1.6 K 0.4 Gd 0.98 Dy 0.02 PO 4 f 2 white phosphor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com