Building material and method for preparing calcium sulfate hemihydrates from titanium white waste sulfuric acid
A technology of calcium sulfate hemihydrate and building materials, which is applied in the field of building materials and the preparation of calcium sulfate hemihydrate by using titanium dioxide waste sulfuric acid, can solve the problems of waste of sulfuric acid resources, high processing cost, complicated process, etc., and achieves environmental protection and operability. Strong and economical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
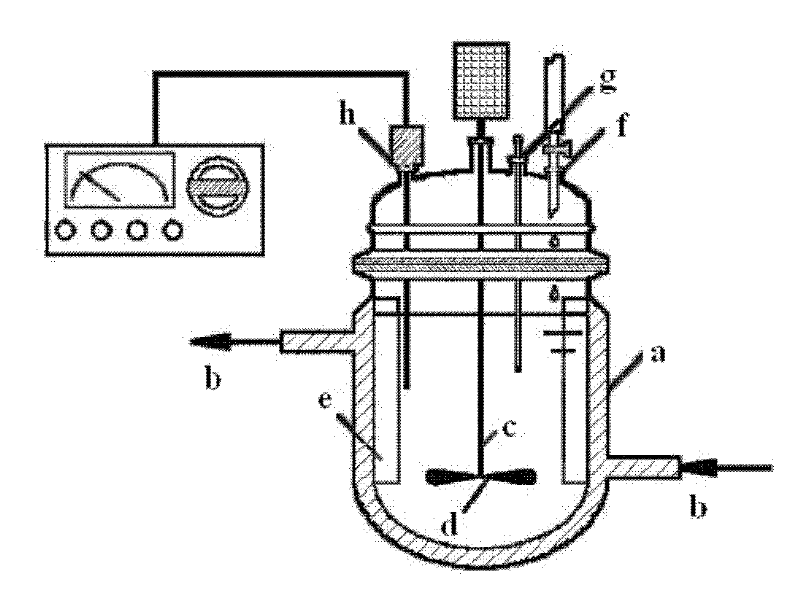
[0037] Such as figure 2 As shown, the jacketed reaction tank used is provided with a circulating water inlet and outlet b on the upper and lower parts of the outer wall of the tank a, one end of the stirring shaft c is connected to the external motor of the tank, and the other end extends into the tank , a stirring blade d is installed on the stirring shaft c in the tank body, four baffles e are fixedly installed on the inner wall of the tank body, and a dripping nozzle f, a thermometer socket g, and a pH meter socket h are opened on the upper part of the tank body .
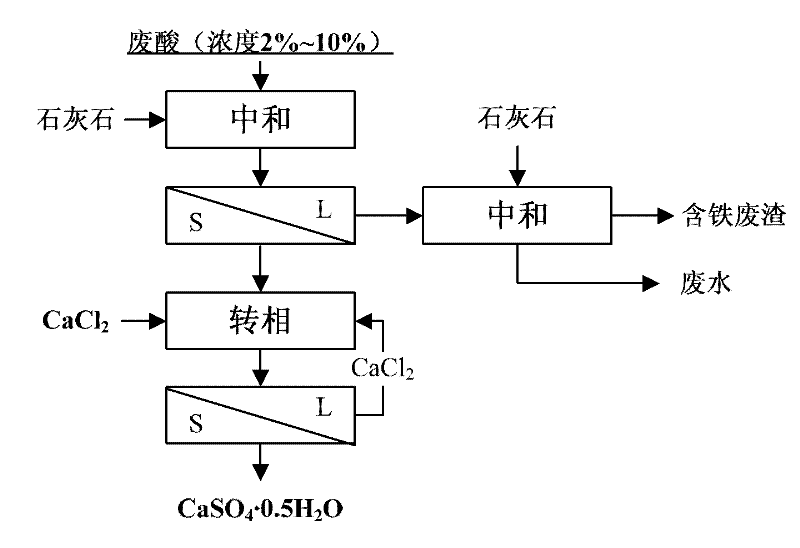
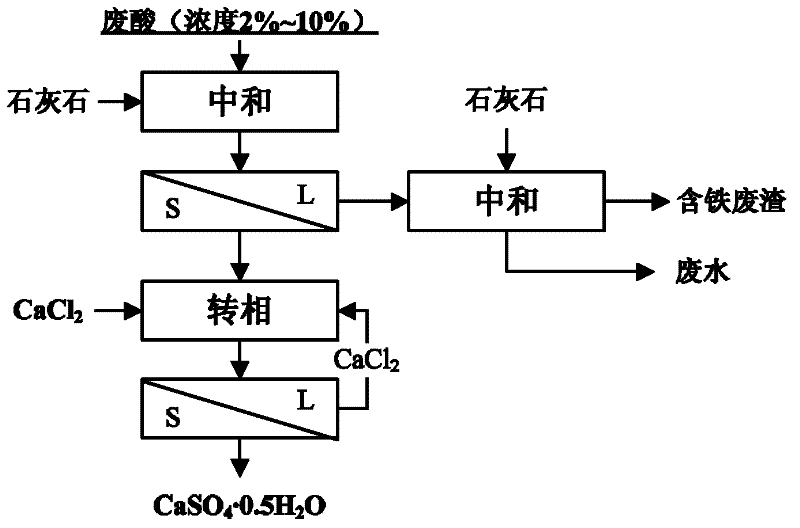
[0038] Process flow of the present invention such as figure 1 As shown, the specific process includes the following steps:
[0039] 1) Add limestone or slaked lime to titanium white waste acid (sulfuric acid with a concentration of 2-10%), stir to let it completely react with sulfuric acid to form calcium sulfate precipitate, and control the pH value to 0.5 to convert the main impurities such as Fe into fine ...
Embodiment 2
[0044] The jacketed reaction tank used in Example 1 was utilized.
[0045] 1) Add limestone or slaked lime to titanium white waste acid (sulfuric acid with a concentration of 2-10%), stir to let it completely react with sulfuric acid to form calcium sulfate precipitate, and control the pH value to 4.5 to convert Fe and other main impurities into fine particles hydroxide precipitation;
[0046] 2) The slurry obtained in step 1) is subjected to solid-liquid separation, the solid phase calcium sulfate dihydrate will be further processed to prepare calcium sulfate hemihydrate, a small amount of lime is added to the liquid phase and natural sedimentation is harmless, and a small amount of solid impurities are piled up to realize calcium sulfate cleaning;
[0047] 3) Add calcium sulfate dihydrate obtained in step 2) into a jacketed reactor, feed circulating water into the jacket of the jacketed reactor, adjust the temperature to 70°C, and add calcium chloride solution , make the mol...
Embodiment 3
[0050] The jacketed reaction tank used in Example 1 was utilized.
[0051] 1) Add limestone or slaked lime to titanium white waste acid (sulfuric acid with a concentration of 2-10%), stir to let it completely react with sulfuric acid to form calcium sulfate precipitate, and control the pH value to 3.0 to convert Fe and other main impurities into fine particles hydroxide precipitation;
[0052] 2) The slurry obtained in step 1) is subjected to solid-liquid separation, the solid phase calcium sulfate dihydrate will be further processed to prepare calcium sulfate hemihydrate, a small amount of lime is added to the liquid phase and natural sedimentation is harmless, and a small amount of solid impurities are stacked to realize calcium sulfate cleaning;
[0053] 3) Add calcium sulfate dihydrate obtained in step 2) into a jacketed reactor, feed circulating water into the jacket of the jacketed reactor, adjust the temperature to 50°C, and add calcium chloride solution , make the mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com