Application of penehyclidine hydrochloride in preparation of medicine for treating motion sickness
A technology for penehyclidine hydrochloride and motion sickness, which is applied in the field of medicine to achieve the effects of broad drug selection space, relieving motion sickness symptoms, and reducing the incidence of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
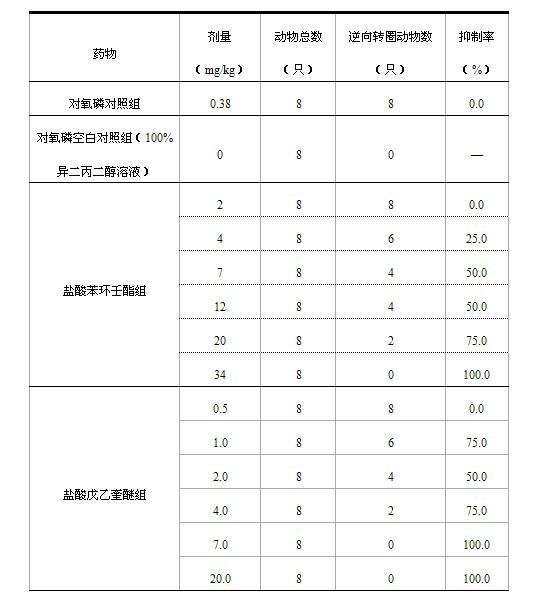
[0028] Example 1 Pharmacodynamic test of penehyclidine hydrochloride against motion sickness in animals
[0029] 1 Abstract Objective To evaluate the prevention and treatment effect of penehyclidine hydrochloride on motion sickness by animal experiments. Methods Reverse circle syndrome in rabbits induced by reversible cholinesterase inhibitors. Conclusion Penehyclidine hydrochloride has obvious anti-motion sickness effect, and the effective dose is significantly lower than that of phencyclonyl hydrochloride.
[0030] 2 materials
[0031] 2.1 Test drugs
[0032] Penehyclidine hydrochloride was synthesized by Chengdu Lisite Pharmaceutical Co., Ltd. with a purity of 99.5%. Paraoxon and isodipropylene glycol were purchased from Acros Company with a purity of more than 99%. Procaine was purchased from Beijing Yongkang Pharmaceutical Co., Ltd. .
[0033] control drug
[0034] Phencynonyl hydrochloride (Phencyclonyl hydrochloride tablets, Beijing Huasu Pharmaceutical Co., Ltd., ...
Embodiment 2
[0051] Example 2 Penehyclidine Hydrochloride Tablets Treated 60 Cases of Motion Sickness
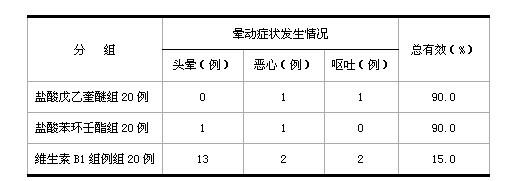
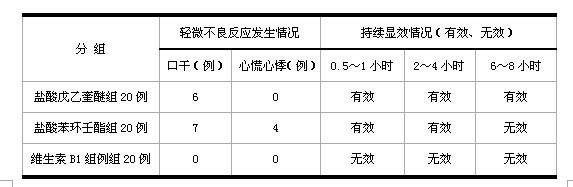
[0052] Randomly select 60 cases of patients who have past medical history of repeated motion sickness and seasickness, and have no heart, liver, lung and blood system abnormalities, frequent headache, insomnia and other symptoms after physical examination, and are divided into three groups, respectively penehyclidine hydrochloride tablet group (1mg / Tablets), phencyclonyl hydrochloride tablet group (2 mg / tablet), vitamin B1 tablet (100 mg / tablet) group, take the medicine 30 minutes before taking the bus, and observe the patients after taking the medicine for 0.5-1 hour, 2-4 hours, 6 to 8 hours, the probability of motion sickness symptoms (dizziness, nausea, vomiting), and the occurrence of adverse reactions such as dry mouth, palpitation and heart palpitations are observed at the same time. The results are shown in Table 2 and Table 3 respectively:
[0053] Table 2 Comparison table of th...
Embodiment 3
[0060] Example 3 Penehyclidine Hydrochloride Patch Used to Relieve Motion Sickness Symptom Test
[0061] Randomly select 20 patients (10 males and 10 females) who have a past medical history of motion sickness and seasickness and have no heart, liver, lung and blood system abnormalities and frequent headaches and insomnia after physical examination. Neiguan point, penehyclidine hydrochloride patch (10mg / tablet), observe the symptoms of motion sickness (dizziness, nausea, vomiting) in 0.5 to 1 hour, 2 to 4 hours, and 6 to 8 hours after the patch is applied. The probability of adverse reactions such as dry mouth, palpitation and heart palpitations were observed at the same time. The results are shown in Table 4:
[0062] Table 4 The total effective rate of penehyclidine hydrochloride patch in relieving motion sickness symptoms
[0063]
[0064] It can be seen that the penehyclidine hydrochloride patch also has a very good therapeutic effect on alleviating motion sickness sym...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com