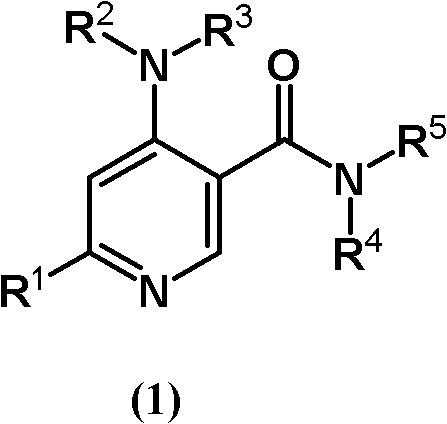
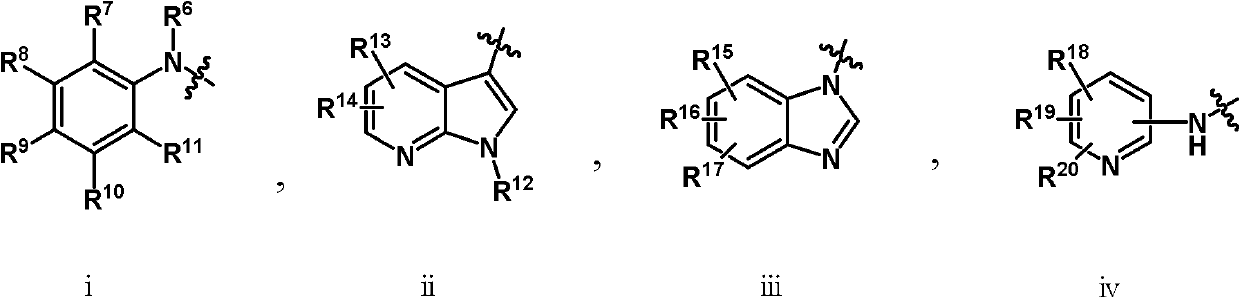
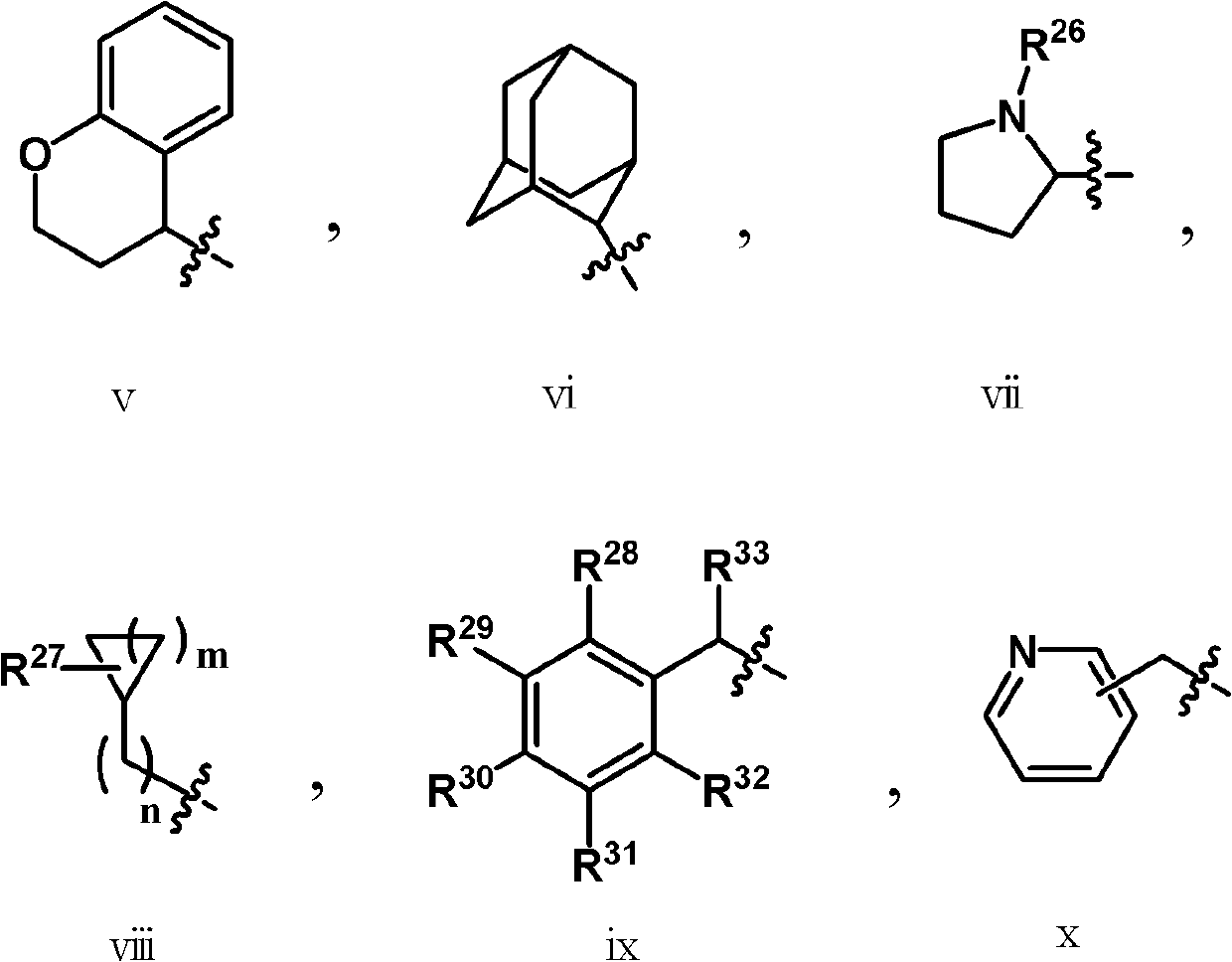
Pyridine-3-carboxyamide derivative
A formamide and derivative technology, applied in the field of pyridine-3-carboxamide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0485] Example 1 Preparation of 4-(benzylamino)-6-chloropyridine-3-carboxamide
[0486]
[0487] Dissolve 5.00 g of 4,6-dichloropyridine-3-carboxamide synthesized according to the method described in US2006 / 0217417 in 50 mL of ethanol, add 3.37 g of benzylamine and 4.40 g of N,N-diisopropylethylamine , heated to reflux for 12 hours. After cooling, the solvent was distilled off, and 100 mL of water was added to the residue. After cooling with ice water, the precipitated crystals were collected by filtration, washed with water and hexane, and air-dried. Furthermore, drying under reduced pressure (100° C., 2 hours) obtained 5.96 g (87%) of the title compound as light yellow needle crystals.
[0488] 1 H-NMR (400MHz, CDCl 3 )δ: 4.42 (2H, d, J=5.6Hz), 5.82 (2H, br), 6.53 (1H, s), 7.26-7.39 (5H, m), 8.28 (1H, s), 8.90 (1H, br )
Embodiment 2
[0489] Example 2 Preparation of 6-chloro-4-[(2-methoxybenzyl)amino]pyridine-3-carboxamide
[0490]
[0491] The title compound was obtained as a colorless crystalline powder from 4,6-dichloropyridine-3-carboxamide and 2-methoxybenzylamine in the same manner as in Example 1 (yield 71%).
[0492] 1 H-NMR (400MHz, CDCl 3 )δ: 3.89 (3H, s), 4.40 (2H, d, J=6.1Hz), 5.77 (2H, br), 6.60 (1H, s), 6.89-6.95 (2H, m), 7.21 (1H, dd , J=8.8, 1.5Hz), 7.29(1H, dd, J=7.6, 1.9Hz), 8.25(1H, s), 8.86(1H, br)
Embodiment 3
[0493] Example 3 Preparation of 6-chloro-4-[(3-methoxybenzyl)amino]pyridine-3-carboxamide
[0494]
[0495] In the same manner as in Example 1, the title compound was obtained as a colorless crystalline powder from 4,6-dichloropyridine-3-carboxamide and 3-methoxybenzylamine (yield 99%).
[0496] 1 H-NMR (400MHz, CDCl 3 )δ: 3.80 (3H, s), 4.39 (2H, d, J=5.6Hz), 5.78 (2H, br), 6.52 (1H, s), 6.82-6.85 (2H, m), 6.88-6.91 (1H , m), 7.27-7.30 (1H, m), 8.28 (1H, s), 8.90 (1H, br)
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com