Medicinal composition for treating blood stasis and qi stagnation syndrome of primary liver cancer and preparation method thereof
A technology of primary liver cancer and composition, which is applied in the direction of drug combination, pharmaceutical formula, medical preparation of non-active ingredients, etc., can solve the problems of energy consumption, difficult filling operation, and affecting protein quality, so as to reduce labor intensity, Reduce the number of freezing and thawing, easy to store for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0043] Experimental example 1: research experiment on excipient change
[0044] 1. Test material:
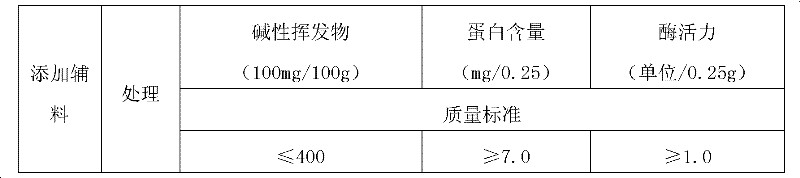
[0045] Ultrafiltrate, β-cyclodextrin, medicinal starch, electronic balance (AUW-120D), electric heating constant temperature drying oven (WK891), electric heating constant temperature water bath (BS60), ultraviolet-visible spectrophotometer (PC-2400), micro Kjeldahl nitrogen analyzer, vacuum pump (SHB-III).
[0046] 2. Methods and Results
[0047] (1) Research on the proportion of excipients added:
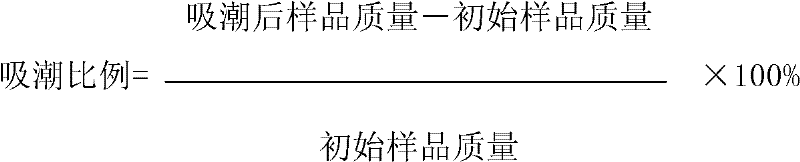
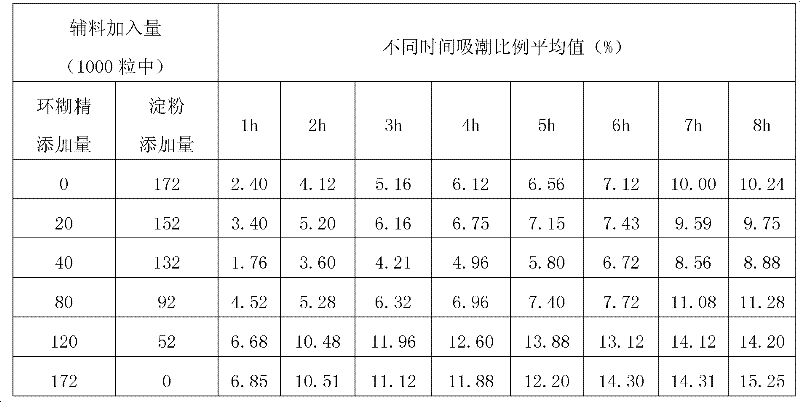
[0048] Add β-cyclodextrin and starch to the ultrafiltrate in a certain proportion. Take 10 liters of ultrafiltrate and add β-cyclodextrin 0g, 20g, 40g, 80g, 120g, 172g respectively, freeze-dry and pulverize, add starch 172g, 152g, 132g, 92g, 52g, 0g in sequence, Dry to obtain each sample. Under the condition of 40% humidity (close to the ambient humidity controlled in the capsule filling production process), the initial sample mass and the mass of the sample exposed to the air ...
experiment example 2
[0063] Experimental example 2: process optimization research
[0064] 1. Research on the amount of water added to the homogenate
[0065] (1) Test materials: paste of fresh white snake, fresh viper and fresh gecko, low-speed centrifuge (SSN600), high-speed centrifuge (GQ-142), ultrafiltration machine (self-made), electronic balance (AUW -120D), electric constant temperature drying oven (WK891), electric constant temperature water bath (BS60), ultraviolet visible spectrophotometer (PC-2400), micro Kjeldahl nitrogen analyzer, vacuum pump (SHB-III).
[0066] (2) Method and results: under the production scale, take 50 kg of the paste and add 1, 2, 4 and 5 times the volume of water, homogenate, then freeze and thaw three times according to the original preparation method, filter, centrifuge, and ultrafilter , record the ultrafiltration speed, the total amount of filtrate, measure the concentration of ultrafiltrate, and calculate the total yield (see Table 3). Repeat the experimen...
Embodiment 1
[0119] Embodiment 1: Capsules
[0120] Fresh Shougong 40kg Fresh Money White Flower Snake 20kg Fresh Viper 20kg
[0121] β-cyclodextrin 1.07kg starch 3.52kg
[0122] Chop fresh gecko, fresh white snake and fresh viper into minced meat, grind into paste, add 4 times the volume of water to homogenate, freeze at -20°C for 24 hours, take it out, and thaw at 25°C. Filtrate, centrifuge the filtrate at 14,000 rpm, absorb the supernatant, ultra-filter, take the filtrate and add β-cyclodextrin inclusions, vacuum freeze-dry, crush into fine particles, add starch, mix well, and dry at low temperature to produce capsules. Take orally, take 3 times a day, take 4 capsules each time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com