Semihydrate crystal of silodosin, preparation method thereof and medicinal composition containing semihydrate crystal
A hemihydrate and crystal technology, which is applied in the direction of drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, to achieve cost-effective, environmentally friendly, low-cost, and easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
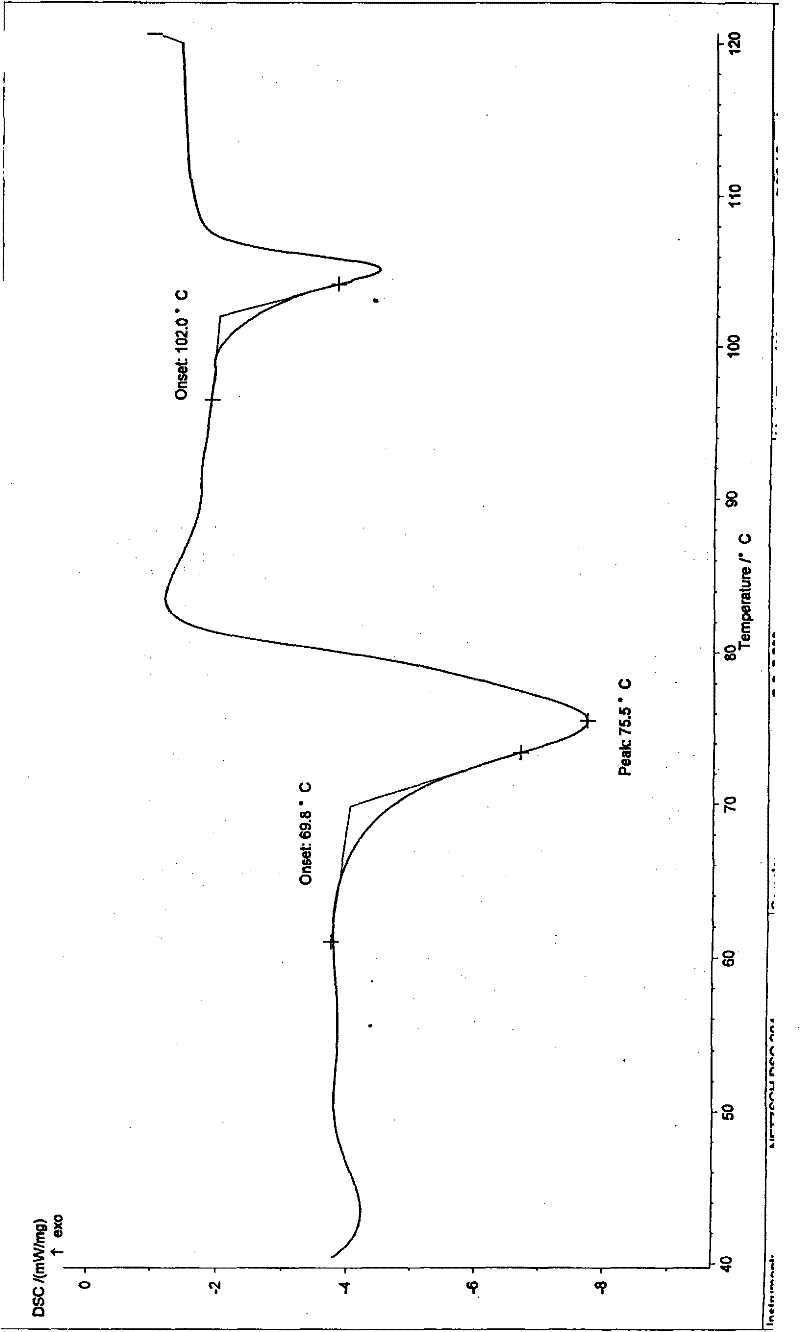
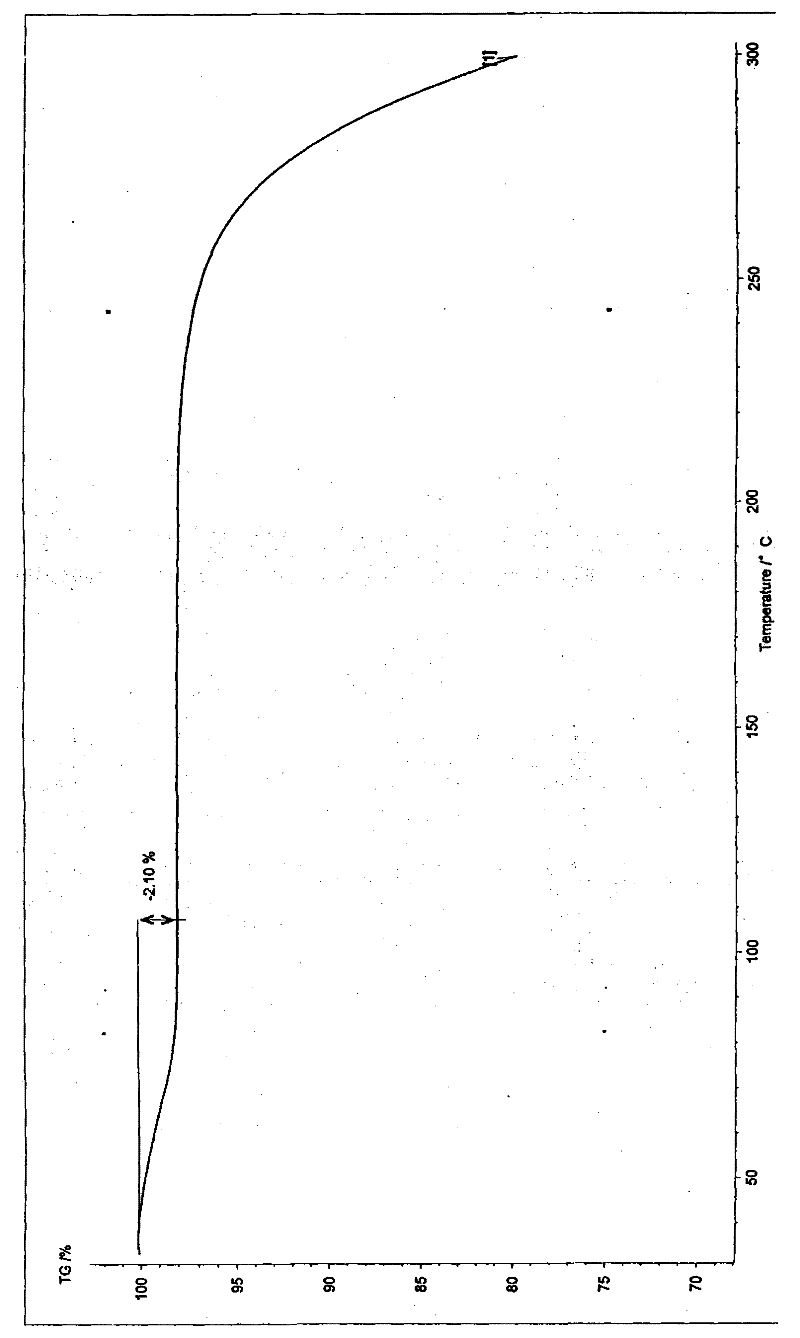
[0027] Implementation example 1: Preparation of hemihydrate crystals of silodosin
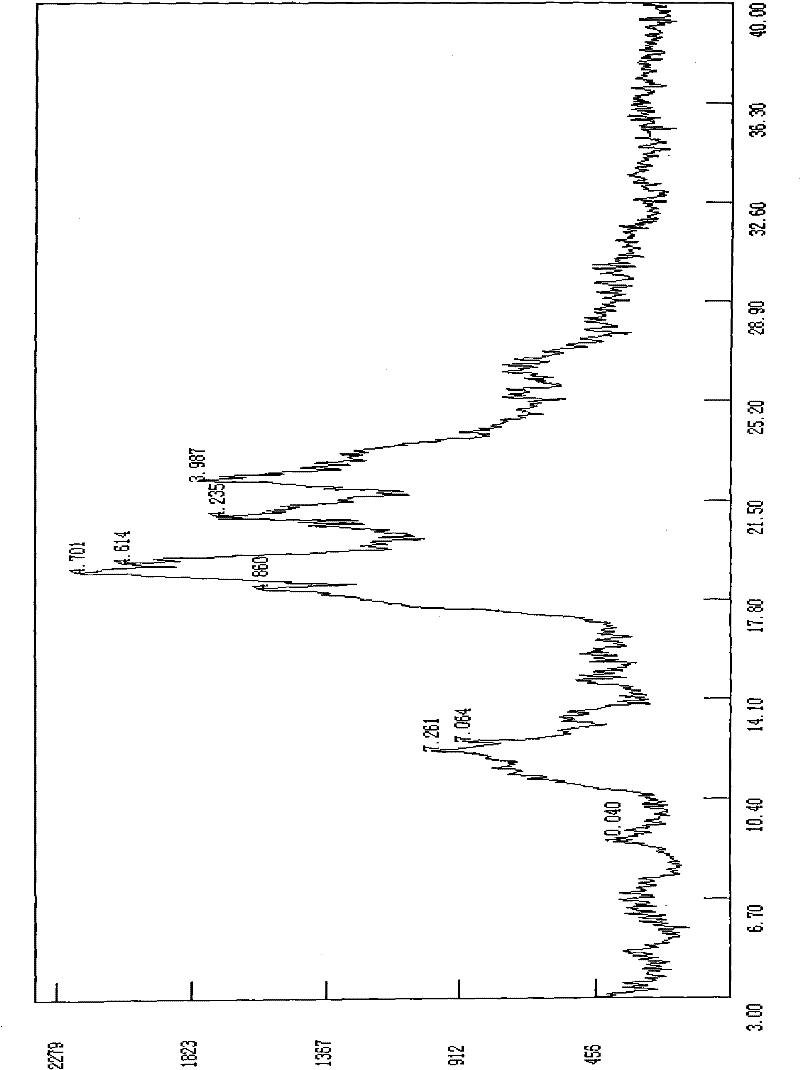
[0028] Take 1g of this product and dissolve it with 2ml of ethanol, filter to remove the insoluble matter, slowly add 30ml of water to the mother liquor under stirring until the crystalline solid is completely precipitated, and continue to stir for 30 minutes. The resulting solid was collected by filtration and dried under vacuum at 50° C. for 12 hours to constant weight to obtain 950 mg of crystals with a purity of 99.3%. The obtained crystalline form is characterized by the following powder X-ray diffraction pattern, which is measured by a diffractometer and expressed in terms of interplanar distance d, 2θ angle, intensity and intensity:
[0029]
Embodiment 2
[0030] Implementation example 2: Preparation of hemihydrate crystals of silodosin
[0031] Take 1g of this product and dissolve it with 2ml of ethyl acetate, filter to remove the insoluble matter, slowly add water dropwise to the mother liquor with stirring until the solid precipitates, add 5ml of water after the solid precipitates, and continue stirring for 0.5 hours. The resulting solid was collected by filtration and dried under vacuum at 50° C. for 12 hours to constant weight to obtain 930 mg of crystals with a purity of 99.5%.
[0032] The obtained crystal form is characterized by the following powder X-ray diffraction pattern, which is measured by a diffractometer and expressed in terms of interplanar distance d, 2θ angle, and intensity:
[0033]
[0034]
Embodiment 3
[0035] Embodiment 3: Capsule 1
[0036] formula:
[0037] Silodosin hemihydrate crystals 2.0g
[0038] Mannitol 66.5g
[0039] Pregelatinized starch 30.0g
[0040] Magnesium Stearate 1.0g
[0041] Sodium Lauryl Sulfate 0.5g
[0042] According to the above formula, 1000 capsules containing 2.0 mg of silodosin hemihydrate crystals were prepared in the capsules by conventional methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com