Preparation method of whole cell suspension of streptococcus pyogenes and serratia marcescens
A technology of Serratia marcescens and Streptococcus pyogenes, which is applied in the field of preparation of whole cell suspensions of Streptococcus pyogenes and Serratia marcescens, can solve the problem that the anti-tumor mechanism is not completely clear and the curative effect is not good , No reports of anti-tumor activity etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
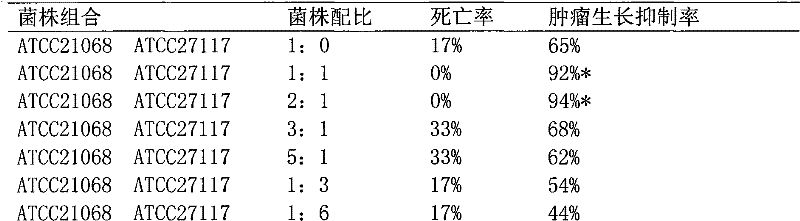
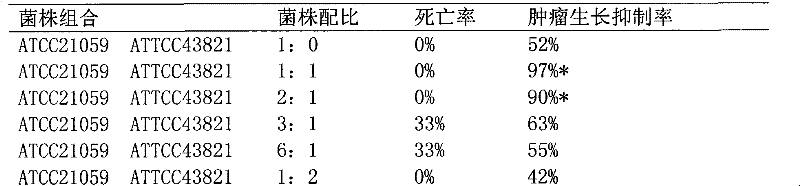
[0033] The preparation of embodiment 1ATCC43820 and ATCC21059 composite bacterial whole cell suspension
[0034] Strains: Streptococcus pyogenes ATCC21059 strain, Serratia marcescens ATCC43820 strain
[0035] Streptococcus pyogenes ATCC21059 strain and Serratia marcescens ATCC43820 strain were cultured separately and mixed in proportion.
[0036] 1. Strain passage
[0037] Medium composition: Each liter of double distilled water contains 3.0 g of beef extract, 10.0 g of Neopeptone (Neopeptone), and 5.0 g of sodium chloride.
[0038] Culture conditions: pH 7.5; 50rpm shaking culture, amplitude 19mm, Serratia marcescens strains were cultured at 25°C; strains of Streptococcus pyogenes were cultured at 37°C.
[0039] Number of passages: the number of passages after the opening of the main seed batch is 1 generation; the number of passages from the opening of the working seed batch to the inoculation of the production medium is 1 generation.
[0040] 2. Preparation of Composite ...
Embodiment 2A
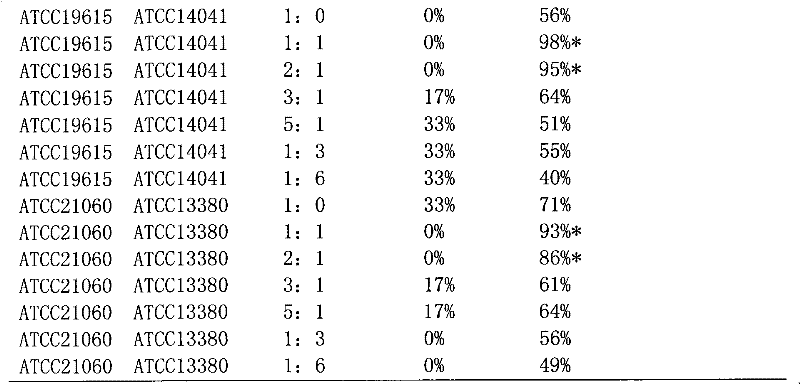
[0044] The preparation of embodiment 2ATTCC43821 and ATCC21060 composite bacterial whole cell suspension
[0045] Strains: Streptococcus pyogenes ATCC21060 strain, Serratia marcescens ATTCC43821 strain
[0046] 1. Strain passage
[0047] Medium composition: Each liter of double distilled water contains 3.0 g of beef extract, 10.0 g of Neopeptone (Neopeptone), and 5.0 g of sodium chloride.
[0048] Culture conditions: pH 7.0; 50rpm shaking culture, amplitude 19mm, Serratia marcescens cultured at 27°C; strain Streptococcus pyogenes cultured at 37°C.
[0049] Number of passages: The number of passages after the main seed batch is opened is 2 generations; the number of passages from the opening of the working seed batch to the inoculation of the production medium is 1 generation.
[0050] 2. Preparation of Composite Bacterial Whole Cell Suspension
[0051] Medium: Each liter of double distilled water contains 3.0 g of beef extract, 10.0 g of Neopeptone, and 5.0 g of sodium chlo...
Embodiment 3A
[0054] The preparation of embodiment 3ATCC13880 and ATCC700924 composite bacterial whole cell suspension
[0055] Strains: Streptococcus pyogenes ATCC700924 strain, Serratia marcescens ATCC13880 strain
[0056] 1. Strain passage
[0057] Medium composition: Each liter of double distilled water contains 3.0 g of beef extract, 10.0 g of Neopeptone (Neopeptone), and 5.0 g of sodium chloride.
[0058] Culture conditions: pH 7.3; 50rpm shaking culture, amplitude 19mm, Serratia marcescens cultured at 25°C; strain Streptococcus pyogenes cultured at 37°C.
[0059] Number of passages: The number of passages after the opening of the main seed batch is 2 generations; the number of passages from the opening of the working seed batch to the inoculation of the production medium is 2 generations.
[0060] 2. Preparation of Composite Bacterial Whole Cell Suspension
[0061] Medium: Each liter of double distilled water contains 3.0 g of beef extract, 10.0 g of Neopeptone, and 5.0 g of sodiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com